

The Impact of COVID-19 on the Careers of Women in Academic Sciences, Engineering, and Medicine (2021)
Chapter: 8 major findings and research questions, 8 major findings and research questions, introduction.
The COVID-19 pandemic, which began in late 2019, created unprecedented global disruption and infused a significant level of uncertainty into the lives of individuals, both personally and professionally, around the world throughout 2020. The significant effect on vulnerable populations, such as essential workers and the elderly, is well documented, as is the devastating effect the COVID-19 pandemic had on the economy, particularly brick-and-mortar retail and hospitality and food services. Concurrently, the deaths of unarmed Black people at the hands of law enforcement officers created a heightened awareness of the persistence of structural injustices in U.S. society.
Against the backdrop of this public health crisis, economic upheaval, and amplified social consciousness, an ad hoc committee was appointed to review the potential effects of the COVID-19 pandemic on women in academic science, technology, engineering, mathematics, and medicine (STEMM) during 2020. The committee’s work built on the National Academies of Sciences, Engineering, and Medicine report Promising Practices for Addressing the Underrepresentation of Women in Science, Engineering, and Medicine: Opening Doors (the Promising Practices report), which presents evidence-based recommendations to address the well-established structural barriers that impede the advancement of women in STEMM. However, the committee recognized that none of the actions identified in the Promising Practices report were conceived within the context of a pandemic, an economic downturn, or the emergence of national protests against structural racism. The representation and vitality of academic women in STEMM had already warranted national attention prior to these events, and the COVID-19
pandemic appeared to represent an additional risk to the fragile progress that women had made in some STEMM disciplines. Furthermore, the future will almost certainly hold additional, unforeseen disruptions, which underscores the importance of the committee’s work.
In times of stress, there is a risk that the divide will deepen between those who already have advantages and those who do not. In academia, senior and tenured academics are more likely to have an established reputation, a stable salary commitment, and power within the academic system. They are more likely, before the COVID-19 pandemic began, to have established professional networks, generated data that can be used to write papers, and achieved financial and job security. While those who have these advantages may benefit from a level of stability relative to others during stressful times, those who were previously systemically disadvantaged are more likely to experience additional strain and instability.
As this report has documented, during 2020 the COVID-19 pandemic had overall negative effects on women in academic STEMM in areas such productivity, boundary setting and boundary control, networking and community building, burnout rates, and mental well-being. The excessive expectations of caregiving that often fall on the shoulders of women cut across career timeline and rank (e.g., graduate student, postdoctoral scholar, non-tenure-track and other contingent faculty, tenure-track faculty), institution type, and scientific discipline. Although there have been opportunities for innovation and some potential shifts in expectations, increased caregiving demands associated with the COVID-19 pandemic in 2020, such as remote working, school closures, and childcare and eldercare, had disproportionately negative outcomes for women.
The effects of the COVID-19 pandemic on women in STEMM during 2020 are understood better through an intentionally intersectional lens. Productivity, career, boundary setting, mental well-being, and health are all influenced by the ways in which social identities are defined and cultivated within social and power structures. Race and ethnicity, sexual orientation, gender identity, academic career stage, appointment type, institution type, age, and disability status, among many other factors, can amplify or diminish the effects of the COVID-19 pandemic for a given person. For example, non-cisgender women may be forced to return to home environments where their gender identity is not accepted, increasing their stress and isolation, and decreasing their well-being. Women of Color had a higher likelihood of facing a COVID-19–related death in their family compared with their white, non-Hispanic colleagues. The full extent of the effects of the COVID-19 pandemic for women of various social identities was not fully understood at the end of 2020.
Considering the relative paucity of women in many STEMM fields prior to the COVID-19 pandemic, women are more likely to experience academic isolation, including limited access to mentors, sponsors, and role models that share gender, racial, or ethnic identities. Combining this reality with the physical isolation stipulated by public health responses to the COVID-19 pandemic,
women in STEMM were subject to increasing isolation within their fields, networks, and communities. Explicit attention to the early indicators of how the COVID-19 pandemic affected women in academic STEMM careers during 2020, as well as attention to crisis responses throughout history, may provide opportunities to mitigate some of the long-term effects and potentially develop a more resilient and equitable academic STEMM system.
MAJOR FINDINGS
Given the ongoing nature of the COVID-19 pandemic, it was not possible to fully understand the entirety of the short- or long-term implications of this global disruption on the careers of women in academic STEMM. Having gathered preliminary data and evidence available in 2020, the committee found that significant changes to women’s work-life boundaries and divisions of labor, careers, productivity, advancement, mentoring and networking relationships, and mental health and well-being have been observed. The following findings represent those aspects that the committee agreed have been substantiated by the preliminary data, evidence, and information gathered by the end of 2020. They are presented either as Established Research and Experiences from Previous Events or Impacts of the COVID-19 Pandemic during 2020 that parallel the topics as presented in the report.
Established Research and Experiences from Previous Events
___________________
1 This finding is primarily based on research on cisgender women and men.
Impacts of the COVID-19 Pandemic during 2020
Research questions.
While this report compiled much of the research, data, and evidence available in 2020 on the effects of the COVID-19 pandemic, future research is still needed to understand all the potential effects, especially any long-term implications. The research questions represent areas the committee identified for future research, rather than specific recommendations. They are presented in six categories that parallel the chapters of the report: Cross-Cutting Themes; Academic Productivity and Institutional Responses; Work-Life Boundaries and Gendered Divisions of Labor; Collaboration, Networking, and Professional Societies; Academic Leadership and Decision-Making; and Mental Health and Well-being. The committee hopes the report will be used as a basis for continued understanding of the impact of the COVID-19 pandemic in its entirety and as a reference for mitigating impacts of future disruptions that affect women in academic STEMM. The committee also hopes that these research questions may enable academic STEMM to emerge from the pandemic era a stronger, more equitable place for women. Therefore, the committee identifies two types of research questions in each category; listed first are those questions aimed at understanding the impacts of the disruptions from the COVID-19 pandemic, followed by those questions exploring the opportunities to help support the full participation of women in the future.
Cross-Cutting Themes
- What are the short- and long-term effects of the COVID-19 pandemic on the career trajectories, job stability, and leadership roles of women, particularly of Black women and other Women of Color? How do these effects vary across institutional characteristics, 2 discipline, and career stage?
2 Institutional characteristics include different institutional types (e.g., research university, liberal arts college, community college), locales (e.g., urban, rural), missions (e.g., Historically Black Colleges and Universities, Hispanic-Serving Institutions, Asian American/Native American/Pacific Islander-Serving Institutions, Tribal Colleges and Universities), and levels of resources.
- How did the confluence of structural racism, economic hardships, and environmental disruptions affect Women of Color during the COVID-19 pandemic? Specifically, how did the murder of George Floyd, Breonna Taylor, and other Black citizens impact Black women academics’ safety, ability to be productive, and mental health?
- How has the inclusion of women in leadership and other roles in the academy influenced the ability of institutions to respond to the confluence of major social crises during the COVID-19 pandemic?
- How can institutions build on the involvement women had across STEMM disciplines during the COVID-19 pandemic to increase the participation of women in STEMM and/or elevate and support women in their current STEMM-related positions?
- How can institutions adapt, leverage, and learn from approaches developed during 2020 to attend to challenges experienced by Women of Color in STEMM in the future?
Academic Productivity and Institutional Responses
- How did the institutional responses (e.g., policies, practices) that were outlined in the Major Findings impact women faculty across institutional characteristics and disciplines?
- What are the short- and long-term effects of faculty evaluation practices and extension policies implemented during the COVID-19 pandemic on the productivity and career trajectories of members of the academic STEMM workforce by gender?
- What adaptations did women use during the transition to online and hybrid teaching modes? How did these techniques and adaptations vary as a function of career stage and institutional characteristics?
- What are examples of institutional changes implemented in response to the COVID-19 pandemic that have the potential to reduce systemic barriers to participation and advancement that have historically been faced by academic women in STEMM, specifically Women of Color and other marginalized women in STEMM? How might positive institutional responses be leveraged to create a more resilient and responsive higher education ecosystem?
- How can or should funding arrangements be altered (e.g., changes in funding for research and/or mentorship programs) to support new ways of interaction for women in STEMM during times of disruption, such as the COVID-19 pandemic?
Work-Life Boundaries and Gendered Divisions of Labor
- How do different social identities (e.g., racial; socioeconomic status; culturally, ethnically, sexually, or gender diverse; immigration status; parents of young children and other caregivers; women without partners) influence the management of work-nonwork boundaries? How did this change during the COVID-19 pandemic?
- How have COVID-19 pandemic-related disruptions affected progress toward reducing the gender gap in academic STEMM labor-force participation? How does this differ for Women of Color or women with caregiving responsibilities?
- How can institutions account for the unique challenges of women faculty with parenthood and caregiving responsibilities when developing effective and equitable policies, practices, or programs?
- How might insights gained about work-life boundaries during the COVID-19 pandemic inform how institutions develop and implement supportive resources (e.g., reductions in workload, on-site childcare, flexible working options)?
Collaboration, Networking, and Professional Societies
- What were the short- and long-term effects of the COVID-19 pandemic-prompted switch from in-person conferences to virtual conferences on conference culture and climate, especially for women in STEMM?
- How will the increase in virtual conferences specifically affect women’s advancement and career trajectories? How will it affect women’s collaborations?
- How has the shift away from attending conferences and in-person networking changed longer-term mentoring and sponsoring relationships, particularly in terms of gender dynamics?
- How can institutions maximize the benefits of digitization and the increased use of technology observed during the COVID-19 pandemic to continue supporting women, especially marginalized women, by increasing accessibility, collaborations, mentorship, and learning?
- How can organizations that support, host, or facilitate online and virtual conferences and networking events (1) ensure open and fair access to participants who face different funding and time constraints; (2) foster virtual connections among peers, mentors, and sponsors; and (3) maintain an inclusive environment to scientists of all backgrounds?
- What policies, practices, or programs can be developed to help women in STEMM maintain a sense of support, structure, and stability during and after periods of disruption?
Academic Leadership and Decision-Making
- What specific interventions did colleges and universities initiate or prioritize to ensure that women were included in decision-making processes during responses to the COVID-19 pandemic?
- How effective were colleges and universities that prioritized equity-minded leadership, shared leadership, and crisis leadership styles at mitigating emerging and potential negative effects of the COVID-19 pandemic on women in their communities?
- What specific aspects of different leadership models translated to more effective strategies to advance women in STEMM, particularly during the COVID-19 pandemic?
- How can examples of intentional inclusion of women in decision-making processes during the COVID-19 pandemic be leveraged to develop the engagement of women as leaders at all levels of academic institutions?
- What are potential “top-down” structural changes in academia that can be implemented to mitigate the adverse effects of the COVID-19 pandemic or other disruptions?
- How can academic leadership, at all levels, more effectively support the mental health needs of women in STEMM?
Mental Health and Well-being
- What is the impact of the COVID-19 pandemic and institutional responses on the mental health and well-being of members of the academic STEMM workforce as a function of gender, race, and career stage?
- How are tools and diagnostic tests to measure aspects of wellbeing, including burnout and insomnia, used in academic settings? How does this change during times of increased stress, such as the COVID-19 pandemic?
- How might insights gained about mental health during the COVID-19 pandemic be used to inform preparedness for future disruptions?
- How can programs that focus on changes in biomarkers of stress and mood dysregulation, such as levels of sleep, activity, and texting patterns, be developed and implemented to better engage women in addressing their mental health?
- What are effective interventions to address the health of women academics in STEMM that specifically account for the effects of stress on women? What are effective interventions to mitigate the excessive levels of stress for Women of Color?
This page intentionally left blank.
The spring of 2020 marked a change in how almost everyone conducted their personal and professional lives, both within science, technology, engineering, mathematics, and medicine (STEMM) and beyond. The COVID-19 pandemic disrupted global scientific conferences and individual laboratories and required people to find space in their homes from which to work. It blurred the boundaries between work and non-work, infusing ambiguity into everyday activities. While adaptations that allowed people to connect became more common, the evidence available at the end of 2020 suggests that the disruptions caused by the COVID-19 pandemic endangered the engagement, experience, and retention of women in academic STEMM, and may roll back some of the achievement gains made by women in the academy to date.
The Impact of COVID-19 on the Careers of Women in Academic Sciences, Engineering, and Medicine identifies, names, and documents how the COVID-19 pandemic disrupted the careers of women in academic STEMM during the initial 9-month period since March 2020 and considers how these disruptions - both positive and negative - might shape future progress for women. This publication builds on the 2020 report Promising Practices for Addressing the Underrepresentation of Women in Science, Engineering, and Medicine to develop a comprehensive understanding of the nuanced ways these disruptions have manifested. The Impact of COVID-19 on the Careers of Women in Academic Sciences, Engineering, and Medicine will inform the academic community as it emerges from the pandemic to mitigate any long-term negative consequences for the continued advancement of women in the academic STEMM workforce and build on the adaptations and opportunities that have emerged.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
Greater Good Science Center • Magazine • In Action • In Education
11 Questions to Ask About COVID-19 Research
Debates have raged on social media, around dinner tables, on TV, and in Congress about the science of COVID-19. Is it really worse than the flu? How necessary are lockdowns? Do masks work to prevent infection? What kinds of masks work best? Is the new vaccine safe?
You might see friends, relatives, and coworkers offer competing answers, often brandishing studies or citing individual doctors and scientists to support their positions. With so much disagreement—and with such high stakes—how can we use science to make the best decisions?
Here at Greater Good , we cover research into social and emotional well-being, and we try to help people apply findings to their personal and professional lives. We are well aware that our business is a tricky one.

Summarizing scientific studies and distilling the key insights that people can apply to their lives isn’t just difficult for the obvious reasons, like understanding and then explaining formal science terms or rigorous empirical and analytic methods to non-specialists. It’s also the case that context gets lost when we translate findings into stories, tips, and tools, especially when we push it all through the nuance-squashing machine of the Internet. Many people rarely read past the headlines, which intrinsically aim to be relatable and provoke interest in as many people as possible. Because our articles can never be as comprehensive as the original studies, they almost always omit some crucial caveats, such as limitations acknowledged by the researchers. To get those, you need access to the studies themselves.
And it’s very common for findings and scientists to seem to contradict each other. For example, there were many contradictory findings and recommendations about the use of masks, especially at the beginning of the pandemic—though as we’ll discuss, it’s important to understand that a scientific consensus did emerge.
Given the complexities and ambiguities of the scientific endeavor, is it possible for a non-scientist to strike a balance between wholesale dismissal and uncritical belief? Are there red flags to look for when you read about a study on a site like Greater Good or hear about one on a Fox News program? If you do read an original source study, how should you, as a non-scientist, gauge its credibility?
Here are 11 questions you might ask when you read about the latest scientific findings about the pandemic, based on our own work here at Greater Good.
1. Did the study appear in a peer-reviewed journal?
In peer review, submitted articles are sent to other experts for detailed critical input that often must be addressed in a revision prior to being accepted and published. This remains one of the best ways we have for ascertaining the rigor of the study and rationale for its conclusions. Many scientists describe peer review as a truly humbling crucible. If a study didn’t go through this process, for whatever reason, it should be taken with a much bigger grain of salt.
“When thinking about the coronavirus studies, it is important to note that things were happening so fast that in the beginning people were releasing non-peer reviewed, observational studies,” says Dr. Leif Hass, a family medicine doctor and hospitalist at Sutter Health’s Alta Bates Summit Medical Center in Oakland, California. “This is what we typically do as hypothesis-generating but given the crisis, we started acting on them.”
In a confusing, time-pressed, fluid situation like the one COVID-19 presented, people without medical training have often been forced to simply defer to expertise in making individual and collective decisions, turning to culturally vetted institutions like the Centers for Disease Control (CDC). Is that wise? Read on.
2. Who conducted the study, and where did it appear?
“I try to listen to the opinion of people who are deep in the field being addressed and assess their response to the study at hand,” says Hass. “With the MRNA coronavirus vaccines, I heard Paul Offit from UPenn at a UCSF Grand Rounds talk about it. He literally wrote the book on vaccines. He reviewed what we know and gave the vaccine a big thumbs up. I was sold.”
From a scientific perspective, individual expertise and accomplishment matters—but so does institutional affiliation.
Why? Because institutions provide a framework for individual accountability as well as safety guidelines. At UC Berkeley, for example , research involving human subjects during COVID-19 must submit a Human Subjects Proposal Supplement Form , and follow a standard protocol and rigorous guidelines . Is this process perfect? No. It’s run by humans and humans are imperfect. However, the conclusions are far more reliable than opinions offered by someone’s favorite YouTuber .
Recommendations coming from institutions like the CDC should not be accepted uncritically. At the same time, however, all of us—including individuals sporting a “Ph.D.” or “M.D.” after their names—must be humble in the face of them. The CDC represents a formidable concentration of scientific talent and knowledge that dwarfs the perspective of any one individual. In a crisis like COVID-19, we need to defer to that expertise, at least conditionally.
“If we look at social media, things could look frightening,” says Hass. When hundreds of millions of people are vaccinated, millions of them will be afflicted anyway, in the course of life, by conditions like strokes, anaphylaxis, and Bell’s palsy. “We have to have faith that people collecting the data will let us know if we are seeing those things above the baseline rate.”
3. Who was studied, and where?
Animal experiments tell scientists a lot, but their applicability to our daily human lives will be limited. Similarly, if researchers only studied men, the conclusions might not be relevant to women, and vice versa.
Many psychology studies rely on WEIRD (Western, educated, industrialized, rich and democratic) participants, mainly college students, which creates an in-built bias in the discipline’s conclusions. Historically, biomedical studies also bias toward gathering measures from white male study participants, which again, limits generalizability of findings. Does that mean you should dismiss Western science? Of course not. It’s just the equivalent of a “Caution,” “Yield,” or “Roadwork Ahead” sign on the road to understanding.
This applies to the coronavirus vaccines now being distributed and administered around the world. The vaccines will have side effects; all medicines do. Those side effects will be worse for some people than others, depending on their genetic inheritance, medical status, age, upbringing, current living conditions, and other factors.
For Hass, it amounts to this question: Will those side effects be worse, on balance, than COVID-19, for most people?
“When I hear that four in 100,000 [of people in the vaccine trials] had Bell’s palsy, I know that it would have been a heck of a lot worse if 100,000 people had COVID. Three hundred people would have died and many others been stuck with chronic health problems.”
4. How big was the sample?
In general, the more participants in a study, the more valid its results. That said, a large sample is sometimes impossible or even undesirable for certain kinds of studies. During COVID-19, limited time has constrained the sample sizes.
However, that acknowledged, it’s still the case that some studies have been much larger than others—and the sample sizes of the vaccine trials can still provide us with enough information to make informed decisions. Doctors and nurses on the front lines of COVID-19—who are now the very first people being injected with the vaccine—think in terms of “biological plausibility,” as Hass says.
Did the admittedly rushed FDA approval of the Pfizer-BioNTech vaccine make sense, given what we already know? Tens of thousands of doctors who have been grappling with COVID-19 are voting with their arms, in effect volunteering to be a sample for their patients. If they didn’t think the vaccine was safe, you can bet they’d resist it. When the vaccine becomes available to ordinary people, we’ll know a lot more about its effects than we do today, thanks to health care providers paving the way.
5. Did the researchers control for key differences, and do those differences apply to you?
Diversity or gender balance aren’t necessarily virtues in experimental research, though ideally a study sample is as representative of the overall population as possible. However, many studies use intentionally homogenous groups, because this allows the researchers to limit the number of different factors that might affect the result.
While good researchers try to compare apples to apples, and control for as many differences as possible in their analyses, running a study always involves trade-offs between what can be accomplished as a function of study design, and how generalizable the findings can be.

The Science of Happiness
What does it take to live a happier life? Learn research-tested strategies that you can put into practice today. Hosted by award-winning psychologist Dacher Keltner. Co-produced by PRX and UC Berkeley’s Greater Good Science Center.
- Apple Podcasts
- Google Podcasts
You also need to ask if the specific population studied even applies to you. For example, when one study found that cloth masks didn’t work in “high-risk situations,” it was sometimes used as evidence against mask mandates.
However, a look beyond the headlines revealed that the study was of health care workers treating COVID-19 patients, which is a vastly more dangerous situation than, say, going to the grocery store. Doctors who must intubate patients can end up being splattered with saliva. In that circumstance, one cloth mask won’t cut it. They also need an N95, a face shield, two layers of gloves, and two layers of gown. For the rest of us in ordinary life, masks do greatly reduce community spread, if as many people as possible are wearing them.
6. Was there a control group?
One of the first things to look for in methodology is whether the population tested was randomly selected, whether there was a control group, and whether people were randomly assigned to either group without knowing which one they were in. This is especially important if a study aims to suggest that a certain experience or treatment might actually cause a specific outcome, rather than just reporting a correlation between two variables (see next point).
For example, were some people randomly assigned a specific meditation practice while others engaged in a comparable activity or exercise? If the sample is large enough, randomized trials can produce solid conclusions. But, sometimes, a study will not have a control group because it’s ethically impossible. We can’t, for example, let sick people go untreated just to see what would happen. Biomedical research often makes use of standard “treatment as usual” or placebos in control groups. They also follow careful ethical guidelines to protect patients from both maltreatment and being deprived necessary treatment. When you’re reading about studies of masks, social distancing, and treatments during the COVID-19, you can partially gauge the reliability and validity of the study by first checking if it had a control group. If it didn’t, the findings should be taken as preliminary.
7. Did the researchers establish causality, correlation, dependence, or some other kind of relationship?
We often hear “Correlation is not causation” shouted as a kind of battle cry, to try to discredit a study. But correlation—the degree to which two or more measurements seem connected—is important, and can be a step toward eventually finding causation—that is, establishing a change in one variable directly triggers a change in another. Until then, however, there is no way to ascertain the direction of a correlational relationship (does A change B, or does B change A), or to eliminate the possibility that a third, unmeasured factor is behind the pattern of both variables without further analysis.
In the end, the important thing is to accurately identify the relationship. This has been crucial in understanding steps to counter the spread of COVID-19 like shelter-in-place orders. Just showing that greater compliance with shelter-in-place mandates was associated with lower hospitalization rates is not as conclusive as showing that one community that enacted shelter-in-place mandates had lower hospitalization rates than a different community of similar size and population density that elected not to do so.
We are not the first people to face an infection without understanding the relationships between factors that would lead to more of it. During the bubonic plague, cities would order rodents killed to control infection. They were onto something: Fleas that lived on rodents were indeed responsible. But then human cases would skyrocket.
Why? Because the fleas would migrate off the rodent corpses onto humans, which would worsen infection. Rodent control only reduces bubonic plague if it’s done proactively; once the outbreak starts, killing rats can actually make it worse. Similarly, we can’t jump to conclusions during the COVID-19 pandemic when we see correlations.
8. Are journalists and politicians, or even scientists, overstating the result?
Language that suggests a fact is “proven” by one study or which promotes one solution for all people is most likely overstating the case. Sweeping generalizations of any kind often indicate a lack of humility that should be a red flag to readers. A study may very well “suggest” a certain conclusion but it rarely, if ever, “proves” it.
This is why we use a lot of cautious, hedging language in Greater Good , like “might” or “implies.” This applies to COVID-19 as well. In fact, this understanding could save your life.
When President Trump touted the advantages of hydroxychloroquine as a way to prevent and treat COVID-19, he was dramatically overstating the results of one observational study. Later studies with control groups showed that it did not work—and, in fact, it didn’t work as a preventative for President Trump and others in the White House who contracted COVID-19. Most survived that outbreak, but hydroxychloroquine was not one of the treatments that saved their lives. This example demonstrates how misleading and even harmful overstated results can be, in a global pandemic.
9. Is there any conflict of interest suggested by the funding or the researchers’ affiliations?
A 2015 study found that you could drink lots of sugary beverages without fear of getting fat, as long as you exercised. The funder? Coca Cola, which eagerly promoted the results. This doesn’t mean the results are wrong. But it does suggest you should seek a second opinion : Has anyone else studied the effects of sugary drinks on obesity? What did they find?
It’s possible to take this insight too far. Conspiracy theorists have suggested that “Big Pharma” invented COVID-19 for the purpose of selling vaccines. Thus, we should not trust their own trials showing that the vaccine is safe and effective.
But, in addition to the fact that there is no compelling investigative evidence that pharmaceutical companies created the virus, we need to bear in mind that their trials didn’t unfold in a vacuum. Clinical trials were rigorously monitored and independently reviewed by third-party entities like the World Health Organization and government organizations around the world, like the FDA in the United States.
Does that completely eliminate any risk? Absolutely not. It does mean, however, that conflicts of interest are being very closely monitored by many, many expert eyes. This greatly reduces the probability and potential corruptive influence of conflicts of interest.
10. Do the authors reference preceding findings and original sources?
The scientific method is based on iterative progress, and grounded in coordinating discoveries over time. Researchers study what others have done and use prior findings to guide their own study approaches; every study builds on generations of precedent, and every scientist expects their own discoveries to be usurped by more sophisticated future work. In the study you are reading, do the researchers adequately describe and acknowledge earlier findings, or other key contributions from other fields or disciplines that inform aspects of the research, or the way that they interpret their results?

Greater Good’s Guide to Well-Being During Coronavirus
Practices, resources, and articles for individuals, parents, and educators facing COVID-19
This was crucial for the debates that have raged around mask mandates and social distancing. We already knew quite a bit about the efficacy of both in preventing infections, informed by centuries of practical experience and research.
When COVID-19 hit American shores, researchers and doctors did not question the necessity of masks in clinical settings. Here’s what we didn’t know: What kinds of masks would work best for the general public, who should wear them, when should we wear them, were there enough masks to go around, and could we get enough people to adopt best mask practices to make a difference in the specific context of COVID-19 ?
Over time, after a period of confusion and contradictory evidence, those questions have been answered . The very few studies that have suggested masks don’t work in stopping COVID-19 have almost all failed to account for other work on preventing the disease, and had results that simply didn’t hold up. Some were even retracted .
So, when someone shares a coronavirus study with you, it’s important to check the date. The implications of studies published early in the pandemic might be more limited and less conclusive than those published later, because the later studies could lean on and learn from previously published work. Which leads us to the next question you should ask in hearing about coronavirus research…
11. Do researchers, journalists, and politicians acknowledge limitations and entertain alternative explanations?
Is the study focused on only one side of the story or one interpretation of the data? Has it failed to consider or refute alternative explanations? Do they demonstrate awareness of which questions are answered and which aren’t by their methods? Do the journalists and politicians communicating the study know and understand these limitations?
When the Annals of Internal Medicine published a Danish study last month on the efficacy of cloth masks, some suggested that it showed masks “make no difference” against COVID-19.
The study was a good one by the standards spelled out in this article. The researchers and the journal were both credible, the study was randomized and controlled, and the sample size (4,862 people) was fairly large. Even better, the scientists went out of their way to acknowledge the limits of their work: “Inconclusive results, missing data, variable adherence, patient-reported findings on home tests, no blinding, and no assessment of whether masks could decrease disease transmission from mask wearers to others.”
Unfortunately, their scientific integrity was not reflected in the ways the study was used by some journalists, politicians, and people on social media. The study did not show that masks were useless. What it did show—and what it was designed to find out—was how much protection masks offered to the wearer under the conditions at the time in Denmark. In fact, the amount of protection for the wearer was not large, but that’s not the whole picture: We don’t wear masks mainly to protect ourselves, but to protect others from infection. Public-health recommendations have stressed that everyone needs to wear a mask to slow the spread of infection.
“We get vaccinated for the greater good, not just to protect ourselves ”
As the authors write in the paper, we need to look to other research to understand the context for their narrow results. In an editorial accompanying the paper in Annals of Internal Medicine , the editors argue that the results, together with existing data in support of masks, “should motivate widespread mask wearing to protect our communities and thereby ourselves.”
Something similar can be said of the new vaccine. “We get vaccinated for the greater good, not just to protect ourselves,” says Hass. “Being vaccinated prevents other people from getting sick. We get vaccinated for the more vulnerable in our community in addition for ourselves.”
Ultimately, the approach we should take to all new studies is a curious but skeptical one. We should take it all seriously and we should take it all with a grain of salt. You can judge a study against your experience, but you need to remember that your experience creates bias. You should try to cultivate humility, doubt, and patience. You might not always succeed; when you fail, try to admit fault and forgive yourself.
Above all, we need to try to remember that science is a process, and that conclusions always raise more questions for us to answer. That doesn’t mean we never have answers; we do. As the pandemic rages and the scientific process unfolds, we as individuals need to make the best decisions we can, with the information we have.
This article was revised and updated from a piece published by Greater Good in 2015, “ 10 Questions to Ask About Scientific Studies .”
About the Authors
Jeremy Adam Smith
Uc berkeley.
Jeremy Adam Smith edits the GGSC’s online magazine, Greater Good . He is also the author or coeditor of five books, including The Daddy Shift , Are We Born Racist? , and (most recently) The Gratitude Project: How the Science of Thankfulness Can Rewire Our Brains for Resilience, Optimism, and the Greater Good . Before joining the GGSC, Jeremy was a John S. Knight Journalism Fellow at Stanford University.
Emiliana R. Simon-Thomas
Emiliana R. Simon-Thomas, Ph.D. , is the science director of the Greater Good Science Center, where she directs the GGSC’s research fellowship program and serves as a co-instructor of its Science of Happiness and Science of Happiness at Work online courses.
You May Also Enjoy

Why Is COVID-19 Killing So Many Black Americans?

How to Keep the Greater Good in Mind During the Coronavirus Outbreak

How Does COVID-19 Affect Trust in Government?

Why Your Sacrifices Matter During the Pandemic

In a Pandemic, Elbow Touches Might Keep Us Going

How to Form a Pandemic Pod
- Research Highlight
- Open access
- Published: 16 March 2020
SARS-CoV-2 and COVID-19: The most important research questions
- Kit-San Yuen 1 ,
- Zi -Wei Ye 2 ,
- Sin-Yee Fung 1 ,
- Chi-Ping Chan 1 &
- Dong-Yan Jin ORCID: orcid.org/0000-0002-2778-3530 1
Cell & Bioscience volume 10 , Article number: 40 ( 2020 ) Cite this article
86k Accesses
381 Citations
170 Altmetric
Metrics details
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an ongoing global health emergency. Here we highlight nine most important research questions concerning virus transmission, asymptomatic and presymptomatic virus shedding, diagnosis, treatment, vaccine development, origin of virus and viral pathogenesis.
The 2019-nCoV causes an ongoing outbreak of lower respiratory tract disease called novel coronavirus pneumonia (NCP) by the Chinese government initially. The disease name was subsequently recommended as COVID-19 by the World Health Organization. Meanwhile, 2019-nCoV was renamed SARS-CoV-2 by the International Committee on Taxonomy of Viruses. As of February 24, 2020, more than 80,000 confirmed cases including more than 2,700 deaths have been reported worldwide, affecting at least 37 countries. The WHO has declared this a global health emergency at the end of January 2020. The epicenter of this ongoing outbreak is in the city of Wuhan in Hubei Province of central China and the Huanan seafood wholesale market was thought to be at least one of the places where SARS-CoV-2 from an unknown animal source might have crossed the species barrier to infect humans.
A pioneering study conducted in the city of Shenzhen near Hong Kong by a group of clinicians and scientists from the University of Hong Kong has provided the first concrete evidence for human-to-human transmission of SARS-CoV-2 [ 1 ]. This is an excellent example of how a high-quality clinical study can make a major difference in policy setting. Several important clinical features of COVID-19 have also been documented in this study. First, an attack rate of 83% within the family context is alarmingly high, indicating the high transmissibility of SARS-CoV-2. Second, the clinical manifestations of COVID-19 in this family range from mild to moderate, with more systematic symptoms and more severe radiological abnormalities seen in older patients. Generally, COVID-19 appears to be less severe than SARS. Third, an asymptomatic child was found to have ground-glass opacities in his lung and SARS-CoV-2 RNA in his sputum sample. This finding of asymptomatic virus shedding raises the possibility for transmission of SARS-CoV-2 from asymptomatic carriers to others, which is later confirmed by others [ 2 ]. Finally, the presentation of diarrhea in two young adults from the same family also suggests the possibility for gastrointestinal involvement in SARS-CoV-2 infection and fecal–oral transmission. The study has set the stage for the control and management of COVID-19 [ 1 ]. The work was completed timely and the investigators showed great courage and leadership in a very difficult time when the Chinese authority failed to recognize widespread person-to-person transmission of SARS-CoV-2 before January 20, 2020.
Several interesting papers on SARS-CoV-2 and COVID-19 have been published in the past few weeks to report on the evolutionary reservoir [ 3 ], possible intermediate host [ 4 ] and genomic sequence [ 5 ] of SARS-CoV-2 as well as clinical characteristics of COVID-19 [ 6 , 7 ]. In view of these findings and the urgent needs in the prevention and control of SARS-CoV-2 and COVID-19, in this commentary we highlight the most important research questions in the field from our personal perspectives.
The first question concerns how SARS-CoV-2 is transmitted currently in the epicenter of Wuhan. In order to minimize the spreading of SARS-CoV-2, China has locked down Wuhan and nearby cities since January 23, 2020. The unprecedented control measures including suspension of all urban transportation have apparently been successful in preventing further spreading of SARS-CoV-2 to other cities. However, the number of confirmed cases in Wuhan continued to rise. It is therefore crucial to determine whether the rise is due to a large number of infected individuals before the lock down and/or failure in the prevention of widespread intra-familial, nosocomial or community transmission. Based on the number of exported cases from Wuhan to cities outside of mainland China, it was predicted that there might be more than 70,000 individuals infected with SARS-CoV-2 on January 25, 2020 in Wuhan [ 8 ]. This should be determined experimentally in Wuhan as discussed below and it will reveal whether the real numbers of infected people and asymptomatic carriers are indeed underestimated severely. In addition to viral RNA detection, measurement of IgM and IgG antibodies as well as antigens would be very helpful. Several representative residential areas should be selected for detailed analysis so that a big picture can be deduced. The analysis should include all healthy and diseased individuals within the area with the aim of identifying people who have recovered from an infection or are having an active infection. The ratio of asymptomatic carriers should also be determined. The analysis should also be extended to detect RNA and antigen of influenza viruses. The activity of seasonal flu in Wuhan also reached a peak at the beginning of 2020. It will be of interest to see whether the flu season had ended and how many people having a fever now are actually infected with influenza virus. Precision control measures for SARS-CoV-2 should be tailor-designed for high-risk groups based on the results of this analysis. Differentiating people having a flu and preventing them from infecting with SARS-CoV-2 in a hospital setting might also be critical.
The second question is how transmissible and pathogenic is SARS-CoV-2 in tertiary and quaternary spreading within humans. Continued transmission of SARS-CoV-2 in Wuhan suggests that tertiary and quaternary spreading has occurred. Compared to the primary and secondary spreading during which SARS-CoV-2 was transmitted from animal to human and from human to human, has the transmission rate increased and has the pathogenicity decreased? Alternatively, is the virus less transmissible after several passages in humans? Retrospective analysis of all confirmed cases in Wuhan should be very informative. The answers to the above questions hold the key to the outcome of the outbreak. If the transmission is weakened, the outbreak may ultimately come to an end at which SARS-CoV-2 is eradicated from humans. On the contrary, if effective transmission can be sustained, the chance is increased that SARS-CoV-2 will become another community-acquired human coronavirus just like the other four human coronaviruses (229E, OC43, HKU1 and NL63) causing common cold only. The basic reproductive number (R 0 ) of SARS-CoV-2 has been estimated to be 2.68, resulting in an epidemic doubling time of about 6.4 days [ 8 ]. Other estimates of R 0 could go up to 4, higher than that of SARS-CoV, which is lower than 2. Determining the real R 0 will shed light on whether and to what extent infection control measures are effective.
The third question relates to the importance of asymptomatic and presymptomatic virus shedding in SARS-CoV-2 transmission. Asymptomatic and presymptomatic virus shedding posts a big challenge to infection control [ 1 , 2 ]. In addition, patients with mild and unspecific symptoms are also difficult to identify and quarantine. Notably, the absence of fever in SARS-CoV-2 infection (12.1%) is more frequent than in SARS-CoV (1%) and Middle East respiratory syndrome coronavirus (MERS-CoV; 2%) infection [ 6 ]. In light of this, the effectiveness of using fever detection as the surveillance method should be reviewed. However, based on previous studies of influenza viruses and community-acquired human coronaviruses, the viral loads in asymptomatic carriers are relatively low [ 9 ]. If this is also the case for SARS-CoV-2, the risk should remain low. Studies on the natural history of SARS-CoV-2 infection in humans are urgently needed. Identifying a cohort of asymptomatic carriers in Wuhan and following their viral loads, clinical presentations and antibody titers over a time course will provide clues as to how many of the subjects have symptoms in a later phase, whether virus shedding from the subjects is indeed less robust, and how often they might transmit SARS-CoV-2 to others.
The fourth question relates to the importance of fecal–oral route in SARS-CoV-2 transmission. In addition to transmission via droplets and close contact, fecal–oral transmission of SARS-CoV has been shown to be important in certain circumstances. Gastrointestinal involvement of SARS-CoV-2 infection and isolation of SARS-CoV-2 from fecal samples of patients are in support of the importance of fecal–oral route in SARS-CoV-2 transmission. Although diarrhea was rarely seen in studies with large cohorts [ 6 , 7 ], the possibility of SARS-CoV-2 transmission via sewage, waste, contaminated water, air condition system and aerosols cannot be underestimated, particularly in cases such as the Diamond Princess cruise ship with 3,700 people, among whom at least 742 have been confirmed to be infected with SARS-CoV-2 plausibly as the result of a superspreading event. Further investigations are required to determine the role of fecal–oral transmission in these cases and within the representative residential areas selected for detailed epidemiological studies in Wuhan as discussed earlier.
The fifth question concerns how COVID-19 should be diagnosed and what diagnostic reagents should be made available. RT-PCR-based SARS-CoV-2 RNA detection in respiratory samples provides the only specific diagnostic test at the initial phase of the outbreak. It has played a very critical role in early detection of patients infected with SARS-CoV-2 outside of Wuhan, implicating that widespread infection of the virus had occurred in Wuhan at least as early as the beginning of 2020. This has also pushed the Chinese authority to acknowledge the severity of the situation. Due to difficulties in sampling and other technical issues in this test, at one point in early February clinically diagnosed patients with typical ground glass lung opacities in chest CT were also counted as confirmed cases in order to have the patients identified and quarantined as early as possible. ELISA kits for detection of IgM and IgG antibodies against N and other SARS-CoV-2 proteins have also been available more recently. This has made specific diagnosis of ongoing and past infection possible. Particularly, seroconversion for IgM antibodies normally occurs a few days earlier than that of IgG. ELISA reagents for detection of SARS-CoV-2 antigens such as S and N are still in urgent need, and would provide another test highly complementary to viral RNA detection.
The sixth question concerns how COVID-19 should be treated and what treatment options should be made available. COVID-19 is a self-limiting disease in more than 80% of patients. Severe pneumonia occurred in about 15% of cases as revealed in studies with large cohorts of patients. The gross case fatality is 3.4% worldwide as of February 25, 2020. This rate is 4.4% for patients in Wuhan, 4.0% for patients in Hubei and 0.92% for patients outside of Hubei. The exceedingly high fatality in Wuhan might be explained by the collapse of hospitals, a large number of undiagnosed patients, suboptimal treatment or a combination of these. Up to date, we still do not have any specific anti-SARS-CoV-2 agents but an anti-Ebola drug, remdesivir, may hold the promise. As a nucleotide analog, remdesivir was shown to be effective in preventing MERS-CoV replication in monkeys. Severity of disease, viral replication, and lung damage were reduced when the drug was administered either before or after infection with MERS-CoV [ 10 ]. These results provide the basis for a rapid test of the beneficial effects of remdesivir in COVID-19. Other antiviral agents worthy of further clinical investigations include ribavirin, protease inhibitors lopinavir and ritonavir, interferon α2b, interferon β, chloroquine phosphate, and Arbidol. However, we should also bear in mind the side effects of these antiviral agents. For example, type I interferons including interferon α2b and interferon β are well known for their antiviral activity. Their beneficial effects at an early phase of infection are well expected. However, administration at a later stage carries the risk that they might worsen the cytokine storm and exacerbate inflammation. Notably, steroids have been experimentally used widely in the treatment of SARS and are still preferred by some Chinese physicians in the treatment of COVID-19. It is said to be capable of stopping the cytokine storm and preventing lung fibrosis. However, the window in which steroids might be beneficial to patients with COVID-19 is very narrow. In other words, steroids can only be used when SARS-CoV-2 has already been eliminated by human immune response. Otherwise, SARS-CoV-2 replication will be boosted leading to exacerbation of symptoms, substantial virus shedding, as well as increased risk for nosocomial transmission and secondary infection. In this regard, it will be of interest to determine whether the report of fungal infection in the lungs of some patients in Wuhan might be linked to misuse of steroids. Nevertheless, the screening of new pharmaceuticals, small-molecule compounds and other agents that have potent anti-SARS-CoV-2 effects will successfully derive new and better lead compounds and agents that might prove useful in the treatment of COVID-19.
The seventh question is whether inactivated vaccines are a viable option for SARS-CoV-2. The chance that SARS-CoV-2 will become endemic in some areas or even pandemic has increased in view of its high transmissibility, asymptomatic and presymptomatic virus shedding, high number of patients with mild symptoms, as well as the evidence for superspreading events. Thus, vaccine development becomes necessary for prevention and ultimate eradication of SARS-CoV-2. Inactivated vaccines are one major type of conventional vaccines that could be easily produced and quickly developed. In this approach, SARS-CoV-2 virions can be chemically and/or physically inactivated to elicit neutralizing antibodies. In the case of SARS-CoV and MERS-CoV, neutralizing antibodies were successfully and robustly induced by an inactivated vaccine in all types of animal experiments, but there are concerns about antibody-dependent enhancement of viral infection and other safety issues. While inactivated vaccines should still be tested, alternative approaches include live attenuated vaccines, subunit vaccines and vectored vaccines. All of these merit further investigations and tests in animals.
The eighth question relates to the origins of SARS-CoV-2 and COVID-19. To make a long story short, two parental viruses of SARS-CoV-2 have now been identified. The first one is bat coronavirus RaTG13 found in Rhinolophus affinis from Yunnan Province and it shares 96.2% overall genome sequence identity with SARS-CoV-2 [ 3 ]. However, RaTG13 might not be the immediate ancestor of SARS-CoV-2 because it is not predicted to use the same ACE2 receptor used by SARS-CoV-2 due to sequence divergence in the receptor-binding domain sharing 89% identity in amino acid sequence with that of SARS-CoV-2. The second one is a group of betacoronaviruses found in the endangered species of small mammals known as pangolins [ 4 ], which are often consumed as a source of meat in southern China. They share about 90% overall nucleotide sequence identity with SARS-CoV-2 but carries a receptor-binding domain predicted to interact with ACE2 and sharing 97.4% identity in amino acid sequence with that of SARS-CoV-2. They are closely related to both SARS-CoV-2 and RaTG13, but apparently they are unlikely the immediate ancestor of SARS-CoV-2 in view of the sequence divergence over the whole genome. Many hypotheses involving recombination, convergence and adaptation have been put forward to suggest a probable evolutionary pathway for SARS-CoV-2, but none is supported by direct evidence. The jury is still out as to what animals might serve as reservoir and intermediate hosts of SARS-CoV-2. Although Huanan seafood wholesale market was suggested as the original source of SARS-CoV-2 and COVID-19, there is evidence for the involvement of other wild animal markets in Wuhan. In addition, the possibility for a human superspreader in the Huanan market has not been excluded. Further investigations are required to shed light on the origins of SARS-CoV-2 and COVID-19.
The ninth question concerns why SARS-CoV-2 is less pathogenic. If the reduced pathogenicity of SARS-CoV-2 is the result of adaptation to humans, it will be of great importance to identify the molecular basis of this adaptation. The induction of a cytokine storm is the root cause of pathogenic inflammation both in SARS and COVID-19. SARS-CoV is known to be exceedingly potent in the suppression of antiviral immunity and the activation of proinflammatory response. It is therefore intriguing to see how SARS-CoV-2 might be different from SARS-CoV in interferon-antagonizing and inflammasome-activating properties. It is noteworthy that some interferon antagonists and inflammasome activators encoded by SARS-CoV are not conserved in SARS-CoV-2. Particularly, ORF3 and ORF8 in SARS-CoV-2 are highly divergent from ORF3a and ORF8b in SARS-CoV that are known to induce NLRP3 inflammasome activation. ORF3 of SARS-CoV-2 is also significantly different from the interferon antagonist ORF3b of SARS-CoV. Thus, these viral proteins of SARS-CoV and SARS-CoV-2 should be compared for their abilities to modulate antiviral and proinflammatory responses. The hypothesis that SARS-CoV-2 might be less efficient in the suppression of antiviral response and the activation of NLRP3 inflammasome should be tested experimentally.
Much progress has been made in the surveillance and control of infectious diseases in China after the outbreak of SARS-CoV in 2003. Meanwhile, virological research in the country has also been strengthened. The new disease report and surveillance system did function relatively well during the 2009 pandemic of swine flu. New viral pathogens such as avian influenza virus H7N9 and severe-fever-with-thrombocytopenia syndrome bunyavirus have also been discovered in recent years [ 11 , 12 ], indicating the strength and vigor of Chinese infectious disease surveillance and virological research. However, the ongoing outbreak of SARS-CoV-2 has not only caused significant morbidity and mortality in China, but also revealed major systematic problems in control and prevention of infectious diseases there. Unfortunately, many of the lessons from the 2003 outbreak have not been learned. Importantly, disease control professionals, practicing physicians and scientists are disconnected in the fight against SARS-CoV-2 and COVID-19. In addition, important decisions were not made by experts in the field. Hopefully, these issues will be dealt with swiftly and decisively during and after the outbreak.
Above we have discussed the two possibilities that this outbreak will unfold. If SARS-CoV-2 is not eliminated from humans through quarantine and other measures, it can still be eradicated by vaccination. If it attenuates to become another community-acquired human coronavirus causing mild respiratory tract disease resembling the other four human coronaviruses associated with common cold, it will not be a disaster either. Before SARS-CoV-2 attenuates further to a much less virulent form, early diagnosis and improved treatment of severe cases hold the key to reduce mortality. We should remain vigilant, but there are grounds for guarded optimism. Redoubling our research efforts on SARS-CoV-2 and COVID-19 will solidify the scientific basis on which important decisions are made.
Availability of data and materials
Not applicable.
Chan JFW, Yuan S, Kok KH, To KKW, Chu H, Yang J, Xing F, Liu J, Yip CCY, Poon RWS, Tsoi HW, Lo SKF, Chan KH, Poon VKM, Chan WM, Ip JD, Cai JP, Cheng VCC, Chen H, Hui CKM, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23.
Article CAS Google Scholar
Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020. https://doi.org/10.1001/jama.2020.1585 .
Article PubMed Google Scholar
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020. https://doi.org/10.1038/s41586-020-2012-7 .
Lam TTY, Shum MHH, Zhu HC, Tong YG, Ni XB, Liao YS, Wei W, Cheung WYM, Li WJ, Li LF, Leung GM, Holmes EC, Hu YL, Guan Y. Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. BioRxiv. 2020. https://doi.org/10.1101/2020.02.13.945485 .
Article Google Scholar
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020. https://doi.org/10.1016/S0140-6736(20)30251-8 .
The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China. China CDC Weekly. 2020;2(8):113–22.
Google Scholar
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019, novel coronavirus–infected pneumonia in Wuhan China. JAMA. 2020. https://doi.org/10.1001/jama.2020.1585 .
Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689-97. https://doi.org/10.1016/S0140-6736(20)30260-9 .
Heimdal I, Moe N, Krokstad S, Christensen A, Skanke LH, Nordbø SA, Døllner H. Human coronavirus in hospitalized children with respiratory tract infections: a 9-year population-based study from Norway. J infect Dis. 2019;219(8):1198–206.
de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. PNAS. 2020. https://doi.org/10.1073/pnas.1922083117 .
Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Chen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang YU, Yuen Z, Shu Y. Human infection with a novel avian-origin influenza virus. N Engl J Med. 2013;368:1888–977.
Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523–32.
Download references
Acknowledgements
We thank Pearl Chan, Hinson Cheung, Terence Lee and Kam-Leung Siu for critical reading of the manuscript.
Coronavirus research in our laboratory was funded by the Hong Kong Health and Medical Research Fund (HKM-15-M01) and Hong Kong Research Grants Council (T11-707/15-R).
Author information
Authors and affiliations.
School of Biomedical Sciences, The University of Hong Kong, 3/F Laboratory Block, 21 Sassoon Road, Pokfulam, Hong Kong
Kit-San Yuen, Sin-Yee Fung, Chi-Ping Chan & Dong-Yan Jin
Department of Microbiology, The University of Hong Kong, Pokfulam, Hong Kong
You can also search for this author in PubMed Google Scholar
Contributions
KSY and DYJ wrote the manuscript with inputs from ZWY, SYF and CPC. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Dong-Yan Jin .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
No potential conflict of interest was reported by the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Yuen, KS., Ye, Z.W., Fung, SY. et al. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci 10 , 40 (2020). https://doi.org/10.1186/s13578-020-00404-4
Download citation
Received : 26 February 2020
Accepted : 07 March 2020
Published : 16 March 2020
DOI : https://doi.org/10.1186/s13578-020-00404-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- 2019 novel coronavirus (2019-nCoV)
- Novel coronavirus pneumonia (NCP)
Cell & Bioscience
ISSN: 2045-3701
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Research on Quantitative Analysis of Multiple Factors Affecting COVID-19 Spread
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected]
Received 2022 Feb 13; Accepted 2022 Mar 4; Collection date 2022 Mar.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
The Corona Virus Disease 2019 (COVID-19) is spreading all over the world. Quantitative analysis of the effects of various factors on the spread of the epidemic will help people better understand the transmission characteristics of SARS-CoV-2, thus providing a theoretical basis for governments to develop epidemic prevention and control strategies. This article uses public data sets from The Center for Systems Science and Engineering at Johns Hopkins University (JHU CSSE), Air Quality Open Data Platform, China Meteorological Data Network, and WorldPop website to construct experimental data. The epidemic situation is predicted by Dual-link BiGRU Network, and the relationship between epidemic spread and various feature factors is quantitatively analyzed by the Gauss-Newton iteration Method. The study found that population density has the greatest positive correlation to the spread of the epidemic among the selected feature factors, followed by the number of landing flights. The number of newly diagnosed daily will increase by 1.08% for every 1% of the population density, the number of newly diagnosed daily will increase by 0.98% for every 1% of the number of landing flights. The results of this study show that the control of social distance and population movement has a high priority in epidemic prevention and control strategies, and it can play a very important role in controlling the spread of the epidemic.
Keywords: quantitative analysis, COVID-19, Gauss-Newton iteration, neural network
1. Introduction
Since December 2019, The Corona Virus Disease 2019 (COVID-19) caused by the SARS-CoV-2, has spread rapidly around the world. On 11 March 2020, the WHO announced that COVID-19 has become a major issue in the world [ 1 , 2 , 3 , 4 ]. The spread of COVID-19 has had a serious impact on the medical and economic aspects of countries around the world [ 5 ]. Due to the complexity of the spread of COVID-19, existing models cannot accurately estimate the direction of the spread of the epidemic [ 6 ]. Therefore, we need to build a quantitative analysis model to deeply explore the spread and influencing factors of COVID-19 on a global scale. In the current research, the data-driven deep learning model has an outstanding performance in the task of modeling time series [ 7 ].
The symptoms of COVID-19 are fever, cough, shortness of breath, loss of consciousness and fatigue. Other symptoms include dyspnea and chest pain [ 8 ]. In order to prevent the spread of the epidemic, countries have adopted many measures, such as reducing gathering activities, controlling the movement of people, advocating the use of masks, and regular disinfection in public areas [ 9 ]. As of 31 December 2021, there have been more than 287 million confirmed cases of COVID-19 worldwide, and at least 5 million people have lost their lives [ 10 ]. In order to further grasp the factors affecting the spread of SARS-CoV-2, better support the decision-making of epidemic prevention and control, timely made targeted countermeasures, and control the further spread of the epidemic, it is very urgent to quantitatively analyze the relationship between various factors and the spread of SARS-CoV-2.
The remainder of this paper is arranged as follows. Section 2 comprehensively introduces the current research on COVID-19 and the transmission characteristics of the SARS-CoV-2. Section 3 introduces the data sources and presents our research methodology. Section 4 describes the experimental results and provides an analytical discussion, and Section 5 summarizes the conclusions of this study and proposes further research directions.
2. Related Research Work
2.1. research on covid-19 epidemic.
Since COVID-19 outbreak in December 2019, research on COVID-19 has attracted the attention of data scientists from all over the world. Duccio et al. [ 11 ] predicted that the maximum number of infections in Italy was about 26,000 and the death toll was about 18,000 through analysis of the spread of the epidemic in China and France. Ricardo et al. [ 12 ] proposed a regression of compressed space Gaussian processes based on chaotic dynamics system to predict the number of people infected with COVID-19 in the United States, and concluded that the number of infected people in the United States would reach more than one million on 14 June 2020. Rohit et al. [ 13 ] proposed Genetic Evolutionary Programming (GEP) to analyze and predict the amount of COVID-19 cases in India. They proposed a GEP model based on the use of a simple function, which was highly effective for the time series prediction of COVID-19 cases in India. Putra et al. [ 14 ] used Particle Swarm Optimization (PSO) to estimate the parameters in the Susceptible Infectives Recovered Model (SIR), and concluded that the parameter results of the PSO algorithm were more accurate and had lower errors than the traditional method. Mbuvha et al. [ 15 ] estimated the parameters of the SIR with data from Lombardy, Italy and Hubei, China, and used the SIR model to predict the number of COVID-19 cases in South Africa, and concluded that COVID-19 was still in the early stage in South Africa.
So far, some scholars have done excellent research, but if it is necessary to further study the transmission characteristics of the SARS-CoV-2, it is impossible to predict the number of patients only. It is necessary to collect data related to the spread of SARS-CoV-2, and to analyze the characteristics of SARS-CoV-2 to understand what factors are related to the spread of SARS-CoV-2 and the quantitative relationship between them, so as to support the more precise adoption of effective prevention, control and disposal measures.
2.2. Research on the Transmission Characteristics of the SARS-CoV-2 Virus
When COVID-19 became a global hot topic, people put forward many speculations that could affect the transmission characteristics of the SARS-CoV-2, such as temperature [ 16 , 17 , 18 ], humidity [ 19 , 20 ], population density [ 21 , 22 ], age [ 23 , 24 ], and so on. In this regard, scholars have also conducted a lot of research, which has a non-negligible inspiration for us to reveal the transmission characteristics of the SARS-CoV-2. Lin et al. [ 25 ] studied the relationship between climate and the spread of COVID-19 on a global scale, and concluded that the spread of COVID-19 was highly correlated with temperature and relative humidity. Roengrudee et al. [ 26 ] studied the relationship between smoking and the spread of COVID-19, and concluded that there was a significant correlation between the number of smokers and the spread of COVID-19. Kass et al. [ 27 ] analyzed the relationship between Body Mass Index (BMI) and age in the number of confirmed COVID-19 patients through a multiple linear regression model, and concluded that obesity may increase the infection rate of COVID-19. WU et al. [ 28 ] found that in the United States, areas with higher historical PM2.5 were positively correlated with higher COVID-19 mortality. Hamit et al. [ 29 ] found that population density was the main factor affecting the spread of the epidemic through research on the spread of the epidemic in Turkish cities.
The above-mentioned studies generally have the following problems: (1) The area covered by the data set is limited to local areas, and the propagation characteristics of SARS-CoV-2 cannot be analyzed from a global scale. (2) The conclusion is only a qualitative analysis, and it has not been able to quantify the effects of various factors on the impact of the spread of the SARS-CoV-2. In response to the above problems, this paper constructs a quantitative analysis model between COVID-19 and multiple factors. Firstly, we collect the required data on a global scale, and then build a Dual-link BiGRU prediction network to predict the number of new cases in each country every day, and quantitatively analyze the impact of different factors on the number of new cases per day of COVID-19. Compared with the above research, the model proposed in this paper is more helpful to analyze the development trend of the epidemic on a global scale, helps to grasp the characteristics of the SARS-CoV-2, and provides more clear theoretical support for the subsequent formulation of anti-epidemic policies by governments of various countries.
3. Data Sources
The data set in this paper is mainly divided into four parts including epidemic data, climate data, population and flight data, and air quality data.
The source of the epidemic data is COVID-19 data set published by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. The data set was collected from all over the world from 22 January 2020, in the early stage of the epidemic. The experimental data in this article include the collected epidemic data from 22 January 2020 to 31 December 2021. The feature data elements include the cumulative number of confirmed cases, the cumulative number of cured people, the cumulative number of deaths, and the number of new cases per day.
The climate data comes from the daily recorded data of weather stations around the world collected by the China Meteorological Data Network ( http://data.cma.cn/ ). This experiment selects the climate data of various regions from 22 January 2020 to 31 December 2021. The feature data elements include daily maximum temperature, daily minimum temperature, wind speed, precipitation, dew point temperature, atmospheric pressure, wind gust, altitude, absolute humidity and relative humidity.
The population and flight data come from the Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat. ( https://population.un.org/wpp/ ). This experiment selects population and flight data in various regions from 22 January 2020 to 31 December 2021. The feature data elements include total population, population density, the total number of flights, number of domestic flights, and international flights.
The air quality data come from the open-source air quality website WAQI ( https://aqicn.org/data-platform/covid19/ ). This experiment selects air quality data in various regions from 22 January 2020 to 31 December 2021. The feature data elements include NO 2 , PM 10 , PM 2.5 , PM 1 , SO 2 , O 3 , CO content in the air, Air Quality Index(AQI), Suspended particle concentration(from NEPH), UV Index(UVI), Pollution(POL) and Wavelength Dominant(WD).
We collected 31-dimensional features of 81 countries to form a data set. Because we can get the data we need in these countries, we selected these 81 countries. In order to ensure that there was a sufficient amount of data to train the model, we selected the 9:1 segmentation ratio to divide the training set and test set, that is, the data from 22 January 2020 to 31 October 2021 was set as the training set and that from 1 November 2021 to 31 December 2021 as the test set.

4. Research Methods
The quantitative relationship model between COVID-19 spread and various characteristic factors proposed in this paper includes three steps: multi-source heterogeneous data preprocessing, constructing Dual-link BiGRU Network to prediction COVID-19 spread, and building a quantitative analysis model of multiple feature data relationships.
4.1. Multi-Source Heterogeneous Data Preprocessing
Because the data comes from a variety of public data sets, there are some problems among data sets, such as inaccurate data, missing data, inconsistent data format and etc. In the data preprocessing stage, this paper builds a dataset with the original data as the core. For inaccurate data, when the values of the same feature data in datasets from different sources are the same, we consider the data to be reasonable; otherwise, most of the data in datasets from different sources are selected as the final data. For missing data, the Cubic Spline Interpolation method is used to supplement the data. For inconsistent data format, feature level fusion method is adopted to extract the features of each source data set first, while the extracted feature information comes from the high-order representation of the original information, and then to aggregate and synthesize the multi-source data according to the feature information. The data with inconsistent scales are normalized by the linear normalization method to unify the data scale. This is also a commonly used data preprocessing method in the field of COVID-19 prediction. The information contained in the fused data is shown in Table 1 .
Feature display of fusion data set.
Tmax, Tmin, Wind_speed, Precipitation, DP_F, Pressure, Wind_gust, Altitude, Ab_humidity and Re_humidity represent daily maximum temperature, daily minimum temperature, daily average wind speed, daily rainfall, daily dew point temperature, atmospheric pressure, wind gust, altitude, absolute humidity and relative humidity. Pop, Density represent total population, population density. NO 2 , PM 10 , PM 2.5 , PM 1 , SO 2 , O 3 , CO and AQI, NEPH, UVI, POL, WD represent NO 2 , PM 10 , PM 2.5 , PM 1 , SO 2 , O 3 , CO content in the air, Air Quality Index(AQI), Suspended particle concentration(from NEPH), UV Index(UVI), Pollution(POL) and Wavelength Dominant(WD). Flight_total, Flight_domestic, and Flight_international represent the total number of flights, the number of domestic flights, and the number of international flights respectively.
4.2. Dual-Link BiGRU Network to Predict the Spread of COVID-19
In this paper, we construct Dual-link BiGRU Network to predict the spread of COVID-19. The task of Dual-link BiGRU is to regress and predict the number of new cases per day with input data. Dual-link BiGRU conducts parameter training through the relationship between daily different factors in the training set and the number of new cases. It inputs the values of the daily factors in the test set, and outputs the regression estimation of the number of new cases on that day. The network structure diagram of Dual-link BiGRU is shown in Figure 1 .
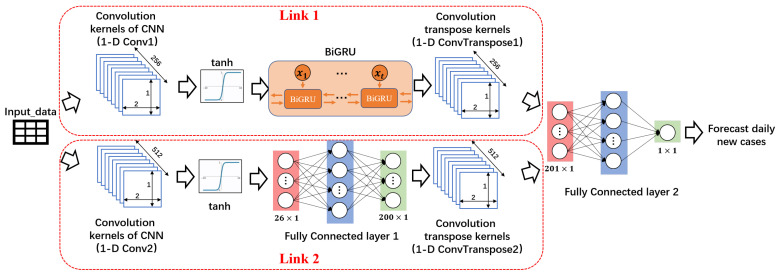
The network structure diagram of Dual-link BiGRU.
Dual-link BiGRU consists of a dual-link feature network and a fully connected network. In the feature network, Link 1 is composed of one-dimensional convolutional network, BiGRU network, and one-dimensional inverse convolutional network. Link 2 is composed of one-dimensional convolutional network, fully connected network, and one-dimensional inverse convolutional network. Link 1 is mainly responsible for learning the timing information in the data of multiple factors. The one-dimensional convolutional network in Link 2 provides a larger receptive field for the network with a larger size of convolution kernel to learn different feature information from Link 1. In this experiment, in order to obtain a larger receptive field and better features, we select the kernel size of 16. After the dual-link feature network is a fully connected network. The fully connected network’s main function is to change the output dimension of the entire Dual-link BiGRU network to the desired output dimension.
According to the prediction performance of the test set, the parameter settings of the prediction network are shown in Table 2 . The optimizer used for model training is Adam, the loss function is Mean Squared Error Loss Function (MSELoss), and the number of iterations is set to 500. In this paper, we selects BiLSTM [ 30 ], BiGRU [ 31 ], and CNN [ 32 ] for comparison at the same dataset which comes from Table 1 . BiLSTM, BiGRU, and CNN are connected by their respective models and fully connected layers. The hidden layer size and number of layers of BiLSTM and BiGRU are consistent with Dual-link BiGRU, and the parameter setting of CNN is consistent with 1-D Conv1 in Dual-link BiGRU.
Prediction network parameter settings.
4.3. The Quantitative Analysis Model of Multi Characteristic Data Relationships
In this paper, we sets the tolerance of the prediction error rate β ∈ [0, 1]. The model with a prediction error rate lower than β is called an effective model, otherwise it is called an invalid model. It is assumed that only effective models can participate in quantitative analysis. Therefore, the larger of β means the more effective models, and the quantitative analysis results have better generalization ability, but it also means that the results have larger errors; the smaller of β means the less effective the models and the poorer generalization ability of the quantitative analysis results, while the results have smaller errors within a limited range. This paper needs to have a small result errors on the basis of ensuring a certain generalization ability, so β = 0.2 is set in the experiment of this paper.
In this paper, the Gauss-Newton iterative method is used for quantitative analysis. The Gauss-Newton iterative method uses Taylor series expansion to approximately replace the nonlinear regression model. Through multiple iterations, the regression coefficient is modified many times, so that the regression coefficient continuously approaches the best regression coefficient of the nonlinear regression model, and finally the Residual Sum of Square of the original model is minimized.
According to the selected observation variable data, a multiple nonlinear regression model as in Equation ( 1 ) can be constructed.
where y is the dependent variable, which represents the number of newly diagnosed people every day in this experiment; X is the set of independent variables, which represents the data of each characteristic factor in this experiment; β is an unknown parameter; ϵ is an error term, and it is an unobservable random variable with a mean of zero and a variance of σ 2 > 0 . The above model can be used to predict the number of the newly diagnosed daily and determine the nonlinear quantitative relationship between each independent variable and the dependent variable. The Gauss-Newton iteration method estimates the to-be-regressed parameter β of the nonlinear regression model through continuous iteration.
The realization process of the quantitative analysis model includes the following steps:
Construct multiple regression models and train through data;
The prediction ability of the model is evaluated by modifying the determination coefficient;
The quantitative relationship between multiple factors and the number of new cases per day was determined by a multiple regression model;
Given different initial values for different factors x 0 ;
For the k t h iteration, calculate the Jacobian matrix J , Hessian matrix H , B , and calculate the increment △ x k ;
If △ x k is small enough, stop the iteration, otherwise, update x ( k + 1 ) = x k + △ x k ;
Repeat steps (5) (6) until the maximum number of iterations is reached, or the termination condition of (6) is met;
Complete the estimation of the unknown parameter β , and determine the quantitative relationship between different elements and the number of new cases per day;
Complete for β to determine the quantitative relationship between different elements and the number of new cases per day.
5. Experimental Results and Discussion
5.1. dual-link bigru.
In this paper, the evaluation index is selected as the error rate ρ , and the error rate calculation formula is shown in Equation ( 2 ):
where y ^ i represents the model output, y i represents the label of the number of new cases per day, and m represents the total number of samples in the test set. This indicator can measure the gap between the model output and the label of the entire test set sample.
In this paper, we selects BiLSTM [ 30 ], BiGRU [ 31 ], and CNN [ 32 ] for comparison at the same dataset which comes from Table 1 . BiLSTM, BiGRU, and CNN are connected by their respective models and fully connected layers. The hidden layer size and number of layers of BiLSTM and BiGRU are consistent with Dual-link BiGRU in Table 2 , and the parameter setting of CNN is consistent with 1-D Conv1 in Dual-link BiGRU in Table 2 . Sets the prediction error tolerance β = 0.2, and uses the model error rate as the evaluation index. In the data of 81 countries, the model with an error rate lower than β is regarded as an effective model, and the difference in the number of effective models among different models is compared in the test dataset. The comparison experiment results are shown in Table 3 .
Comparison of model results.
Table 3 shows that (1) Dual-link BiGRU has a larger effective model ratio in the prediction network; (2) Compared with BiGRU, BiLSTM, and CNN, Dual-link BiGRU performs better in low error rate. Therefore, it is believed that the Dual-link BiGRU has better performance and generalization ability in predicting the daily number of new epidemics in various countries. Therefore, this paper selects the Dual-link BiGRU as the prediction network. Figure 2 shows the difference between the daily number of new cases predicted of the Dual-link BiGRU and the label value. Because showing the forecast results for all countries would make the paper extraordinarily long, in this paper, we select 6 countries with better results for display, including Canada, China, India, Indonesia, Russia, and United Kingdom.
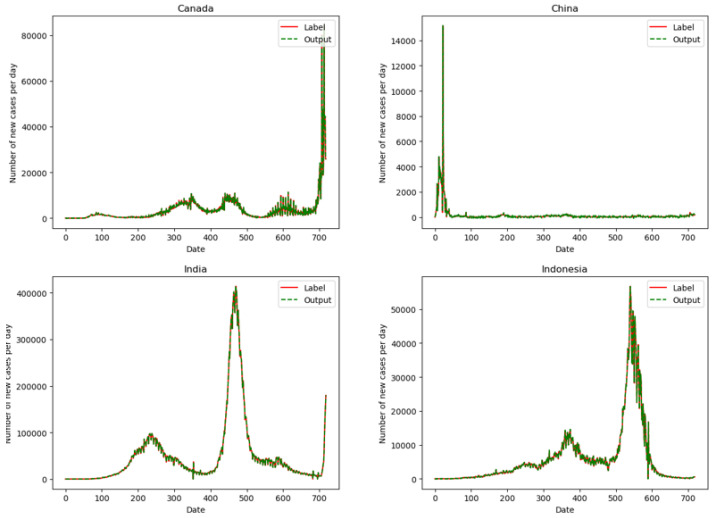
Display of Dual-link BiGRU prediction results.
It can be seen from Figure 2 that in the selected 6 countries, the red solid line is the label of the number of new cases per day, and the green dashed line is the predicted value by the Dual-link BiGRU network. The two curves have a high degree of overlap. Therefore, the prediction network constructed in the experiment has a good fit with the real data. The trained prediction network can better predict the daily new cases and has a strong generalization ability. For different countries, the model can learn more appropriate parameters to predict the number of the daily new cases.
5.2. Quantitative Analysis Results of Multi-Characteristic Data Relationships
In this paper, we uses the method of Lin [ 25 ] and others to build a multiple regression model for the selected 44 effective national models and train them. Through the multiple regression model, the quantitative relationship between multiple factors and the number of new cases per day is determined, and the prediction ability of the model is evaluated by determining the Adjusted R Square (R). The larger R is, the stronger the prediction ability of the model is. If R is greater than 0.6, the model has strong epidemic prediction ability. Then, the initial value of the Gauss-Newton iterative method is selected through the model parameters. The quantitative relationship between multiple factors and the number of new cases per day is shown in Table 4 , and the initial values are shown in Table 5 .
Regression equation parameter.
Example of initial value of each characteristic coefficient.
In this paper, we uses the trained Dual-link BiGRU model of various countries to generate simulation data for quantitative analysis. The data generation method is as follows:
Goal: To generate data for analyzing the quantitative relationship between x 1 and y , where x 1 is the maximum temperature per day and y is the number of new cases per day.
To control other factors unchanged, adjust x 1 , and generate the predicted value of y .
The simulation data is used as input, and training is performed with the Gauss-Newton method to obtain the coefficient between x 1 and y , so as to determine the quantitative relationship between them.
According to the above method, the coefficient equations between the number of new cases per day in each country and the characteristic factors in Table 1 are obtained respectively, and the quantitative relationship between the number of new cases per day and the characteristic factors in each country is determined. Then take the average of the quantitative relationship coefficients of the same feature in all countries, and finally get the quantitative relationship between each feature that is applicable in the selected country and the number of new cases per day with strong generalization performance, as shown in Table 6 .
Quantitative relationship between characteristic factors and daily number of new cases.
The influence >0, indicating that the factor has a positive correlation with the increase in the number of new cases per day. The influence <0, indicating that the factor has a negative correlation with the increase in the number of new cases per day.
As shown in Table 6 , among the selected features, the population density per unit land area has the largest positive correlation with the number of new cases per day, followed by the number of landing flights. The population density per square kilometer increases by 1%, and the number of new cases per day in the corresponding area increases by about 1.076%. For every 1% increase in the number of landing flights, the number of new cases per day in the corresponding area increases by about 0.98%. Among the selected features, the daily maximum temperature, daily minimum temperature and dew point temperature have negative correlations to the number of new cases per day. Within the range of 0–50 °C, each increase of 1 °C can reduce the number of new cases per day by 0.021%, 0.028% and 0.015% respectively.
Based on the above analysis, the following further inferences can be drawn:
Population factors and flight factors has an obvious positive correlation impact on the spread of COVID-19. From the data of the selected 44 countries, it can be seen that population factors and flight factors have a greater impact on the spread of COVID-19. Every 1% increase in population factors will increase the spread of the epidemic by 1.044%. Every 1% increase in the number of arrival flights will increase the spread of the epidemic by 0.98%. Therefore it can be seen that population factors and flight factors have a more obvious impact on the increase in the spread of the epidemic. From the perspective of formulating epidemic prevention and control policies, controlling social distancing and population movement will have a more obvious positive correlation impact on epidemic prevention and control.
The increase in temperature and relative humidity has a negative correlation impact on the spread of COVID-19.Among the climatic factors, the increase of temperature and humidity has a negative correlation impact on the spread of COVID-19. In this paper, the temperature range of 0–50 °C and the relative humidity range of 1–100% are selected for the experiment. It is obtained that within this range, temperature and relative humidity has a negative correlation impact on the spread of COVID-19, but the impact is not obvious. Since the correlation between population density and the speed of the epidemic is far greater than the correlation between temperature and the speed of the epidemic, it is speculated that in areas with higher temperatures and higher population densities, such as India and other countries, the speed of the epidemic still has a relatively rapid possibility.
A larger AQI has a positive correlation impact on the spread of COVID-19.AQI represents the degree of air cleanliness or pollution and its impact on health. The higher the AQI, the more serious the air pollution in the region. This experiment shows that in the range of AQI value 100–200, the epidemic transmission speed of COVID-19 will increase by 0.013% every time AQI increases by 1. Some researchers have shown that SARS-CoV-2 can spread through aerosols [ 33 , 34 , 35 ]. Therefore, a higher AQI means a higher aerosol content in the air, which is not good for air circulation. Such an environment may promote the spread of COVID-19.
6. Discussion
Since the discovery of COVID-19 in 2019, countries have successively formulated epidemic prevention and control policies that suit their own national conditions [ 36 ]. According to the current development status of the world epidemic, a long-term coexistence with the virus has been formed, that is, even though the vaccine has been developed, it will take a long time to completely eliminate COVID-19 [ 37 , 38 ]. This paper carries out quantitative analysis and research on COVID-19 transmission by various factors all over the world and comes to the conclusion that the increase of population density, population flow, and flight times has a positively correlated impact on the epidemic transmission, and the increase of temperature, relative humidity, and dew point temperature has a negative correlation impact on the epidemic transmission. It can be concluded that the positive correlation effect of population density on the epidemic spread is much greater than the negative correlation effect of climate factors on the epidemic spread.
Therefore, according to the regional characteristics and national conditions, governments should formulate epidemic prevention and control policies to control population density and population flow in the climate environment with high local temperature and relative humidity, maximize the effect of epidemic prevention and control, and curb the spread of the epidemic from the aspects of transmission route and virus characteristics.
International organizations need to establish high, medium and low-risk epidemic spread levels globally. The faster the epidemic spread, the higher the epidemic spread level, and the more stringent prevention and control policies need to be adopted. For cities where the epidemic has spread, it is necessary to keep wearing masks, maintain proper social distancing, and reduce public recreational activities. For cities with large population density and serious epidemic spread, it is recommended to strictly control population flow, tighten restrictive measures for international flights, and take “city closure” measures when necessary, and other cities need to take more stringent entry epidemic prevention measures for personnel from high-risk countries and regions. For cities with slow epidemic spread, it is suggested to control the population flow within a certain range, allow international flights under the condition of good epidemic prevention measures, strictly control the flow of people from high-risk countries and regions, and be vigilant against the epidemic spread caused by climate change.
7. Conclusions and Future Work
In this paper, we fuses multi-source heterogeneous data, and makes predictions for the current COVID-19 epidemic based on the fusion data set, and quantitatively analyzes the model to obtain the quantitative relationship between various factors and the spread of the epidemic. The contributions of this paper are as follows:
- Compared with the CNN, LSTM, and GRU networks, the prediction accuracy of the Dual-link BiGRU network is improved by 35.03%, 31.41%, and 27.36%, respectively;
- Compared with the CNN, LSTM, and GRU networks, the generalization ability of the Dual-link BiGRU network is improved by 25.00%, 27.50%, and 28.75%, respectively.
- The increase in population factors and flight factors has an obvious positively correlated impact on the spread of COVID-19.
- The increase in AQI will has a minor positively correlated impact on the spread of COVID-19.
- The increase in temperature and relative humidity has a negative correlation impact on the spread of COVID-19.
Accordingly, this paper makes the following recommendations for global epidemic prevention and control:
Countries should take appropriate or even stricter prevention and control measures according to their national conditions, such as demographic factors, climate factors, air quality factors, and the number of flights, to minimize the risk of outbreaks.
Demographic factors have a strong positive relationship with the spread of COVID-19 epidemic. Governments can control the spread of the epidemic by strictly controlling the movement of people both within and outside the country.
Since the impact of population and flight factors on the spread of the epidemic is much greater than that of climate factors, governments of various countries should not expect the epidemic to disappear after the temperature rises, and should actively control population movement.
This paper has completed the multi-factor quantitative analysis model affecting the spread of COVID-19. Due to the different detection coverage of COVID-19 in various countries, the number of confirmed cases is inevitably underestimated, and this paper does not evaluate the impact of changes in policies and local prevention and control strategies on the spread of COVID-19. Therefore, more detailed exploration is needed on these issues in the next step.
Author Contributions
Y.F. collected literature and wrote the manuscript; S.L. reviewed and edited the manuscript; Z.X. Put forward suggestions for revision of the article. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Yunbo X.S.W. Novel coronavirus pneumonia epidemic and China’s urban population and the spatial relationship between the city’s public health classification governance enlightenment. Trop. Geogr. 2020;40:408–421. [ Google Scholar ]
- 2. Sharifi A., Khavarian-Garmsir A.R. The COVID-19 pandemic: Impacts on cities and major lessons for urban planning, design, and management. Sci. Total Environ. 2020;749:142391. doi: 10.1016/j.scitotenv.2020.142391. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Conforti C., Giuffrida R., Dianzani C., Di Meo N., Zalaudek I. COVID-19 and psoriasis: Is it time to limit treatment with immunosuppressants? A call for action. Dermatol. Ther. 2020;33:e13298. doi: 10.1111/dth.13298. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Arora P., Jafferany M., Lotti T., Sadoughifar R., Goldust M. Learning from history: Coronavirus outbreaks in the past. Dermatol. Ther. 2020;33:e13343. doi: 10.1111/dth.13343. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop. Med. Int. Health. 2020;25:278. doi: 10.1111/tmi.13383. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Fauci A.S., Lane H.C., Redfield R.R. COVID-19—Navigating the Uncharted. N. Engl. J. Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Namasudra S., Dhamodharavadhani S., Rathipriya R. Nonlinear neural network based forecasting model for predicting COVID-19 cases. Neural Process. Lett. 2021:1–21. doi: 10.1007/s11063-021-10495-w. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Watkins J. Preventing a COVID-19 Pandemic. BMJ. 2020;368:m810. doi: 10.1136/bmj.m810. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Haynes B.F., Corey L., Fernandes P., Gilbert P.B., Hotez P.J., Rao S., Santos M.R., Schuitemaker H., Watson M., Arvin A. Prospects for a safe COVID-19 vaccine. Sci. Transl. Med. 2020;12:eabe0948. doi: 10.1126/scitranslmed.abe0948. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Fanelli D., Piazza F. Analysis and forecast of COVID-19 spreading in China, Italy and France. Chaos Solitons Fractals. 2020;134:109761. doi: 10.1016/j.chaos.2020.109761. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Velásquez R.M.A., Lara J.V.M. Forecast and evaluation of COVID-19 spreading in USA with reduced-space Gaussian process regression. Chaos Solitons Fractals. 2020;136:109924. doi: 10.1016/j.chaos.2020.109924. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Rahimi I., Chen F., Gandomi A.H. A review on COVID-19 forecasting models. Neural Comput. Appl. 2021:1–11. doi: 10.1007/s00521-020-05626-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Putra S., Khozin Mutamar Z. Estimation of parameters in the SIR epidemic model using particle swarm optimization. Am. J. Math. Comput. Model. 2019;4:83–93. doi: 10.11648/j.ajmcm.20190404.11. [ DOI ] [ Google Scholar ]
- 15. Mbuvha R.R., Marwala T. On data-driven management of the COVID-19 outbreak in South Africa. medRxiv. 2020 doi: 10.1101/2020.04.07.20057133. [ DOI ] [ Google Scholar ]
- 16. Notari A. Temperature dependence of COVID-19 transmission. Sci. Total Environ. 2021;763:144390. doi: 10.1016/j.scitotenv.2020.144390. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Shi P., Dong Y., Yan H., Zhao C., Li X., Liu W., He M., Tang S., Xi S. Impact of temperature on the dynamics of the COVID-19 outbreak in China. Sci. Total Environ. 2020;728:138890. doi: 10.1016/j.scitotenv.2020.138890. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Tobías A., Molina T. Is temperature reducing the transmission of COVID-19? Environ. Res. 2020;186:109553. doi: 10.1016/j.envres.2020.109553. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Wang J., Tang K., Feng K., Li X., Lv W., Chen K., Wang F. High temperature and high humidity reduce the transmission of COVID-19. arXiv. 2020 doi: 10.2139/ssrn.3551767.2003.05003 [ DOI ] [ Google Scholar ]
- 20. Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S., Yan J., Niu J., Zhou J., Luo B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020;724:138226. doi: 10.1016/j.scitotenv.2020.138226. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Bhadra A., Mukherjee A., Sarkar K. Impact of population density on Covid-19 infected and mortality rate in India. Model. Earth Syst. Environ. 2021;7:623–629. doi: 10.1007/s40808-020-00984-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Sun Z., Zhang H., Yang Y., Wan H., Wang Y. Impacts of geographic factors and population density on the COVID-19 spreading under the lockdown policies of China. Sci. Total Environ. 2020;746:141347. doi: 10.1016/j.scitotenv.2020.141347. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Eggo R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Monod M., Blenkinsop A., Xi X., Hebert D., Bershan S., Tietze S., Baguelin M., Bradley V.C., Chen Y., Coupland H., et al. Age groups that sustain resurging COVID-19 epidemics in the United States. Science. 2021;371:eabe8372. doi: 10.1126/science.abe8372. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Lin S., Fu Y., Jia X., Ding S., Wu Y., Huang Z. Discovering correlations between the COVID-19 epidemic spread and climate. Int. J. Environ. Res. Public Health. 2020;17:7958. doi: 10.3390/ijerph17217958. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Patanavanich R., Glantz S.A. Smoking is associated with COVID-19 progression: A meta-analysis. Nicotine Tob. Res. 2020;22:1653–1656. doi: 10.1093/ntr/ntaa082. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395:1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;6:eabd4049. doi: 10.1126/sciadv.abd4049. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Coşkun H., Yıldırım N., Gündüz S. The spread of COVID-19 virus through population density and wind in Turkey cities. Sci. Total. Environ. 2021;751:141663. doi: 10.1016/j.scitotenv.2020.141663. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Zeroual A., Harrou F., Dairi A., Sun Y. Deep learning methods for forecasting COVID-19 time-Series data: A Comparative study. Chaos Solitons Fractals. 2020;140:110121. doi: 10.1016/j.chaos.2020.110121. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Behura A. A deep learning application for prediction of COVID-19. In: Mishra S., Mallick P.K., Tripathy H.K., Chae G.S., Mishra B.S.P., editors. Impact of AI and Data Science in Response to Coronavirus Pandemic. Springer; Berlin/Heidelberg, Germany: 2021. pp. 127–148. Algorithms for Intelligent Systems. [ Google Scholar ]
- 32. Aslan M.F., Unlersen M.F., Sabanci K., Durdu A. CNN-based transfer learning–BiLSTM network: A novel approach for COVID-19 infection detection. Appl. Soft Comput. 2021;98:106912. doi: 10.1016/j.asoc.2020.106912. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Anderson E.L., Turnham P., Griffin J.R., Clarke C.C. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal. 2020;40:902–907. doi: 10.1111/risa.13500. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010;76:2712–2717. doi: 10.1128/AEM.02291-09. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Gardner E.G., Kelton D., Poljak Z., Van Kerkhove M., Von Dobschuetz S., Greer A.L. A case-crossover analysis of the impact of weather on primary cases of Middle East respiratory syndrome. BMC Infect. Dis. 2019;19:1–10. doi: 10.1186/s12879-019-3729-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Zhou C., Pei T., Du Y., Chen J., Xu J., Wang J., Zhang G., Su F., Song C., Yi J., et al. Big data analysis on COVID-19 epidemic and suggestions on regional prevention and control policy. Bull. Chin. Acad. Sci. 2020;35:200–203. [ Google Scholar ]
- 37. Cheng Z.J., Qu H.Q., Tian L., Duan Z., Hakonarson H. COVID-19: Look to the Future, Learn from the Past. Viruses. 2020;12:1226. doi: 10.3390/v12111226. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Sun C., Zhai Z. The efficacy of social distance and ventilation effectiveness in preventing COVID-19 transmission. Sustain. Cities Soc. 2020;62:102390. doi: 10.1016/j.scs.2020.102390. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (640.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Research article
- Open access
- Published: 04 June 2021
Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
18k Accesses
37 Citations
14 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
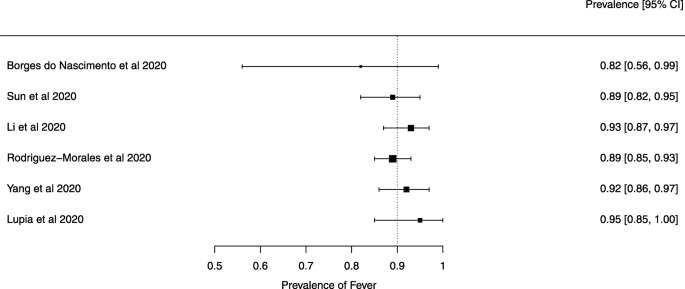
A meta-analysis of the prevalence of fever
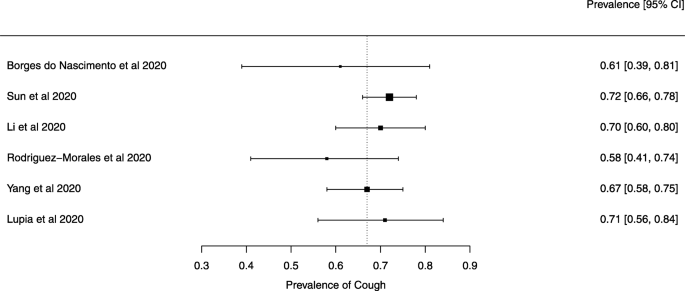
A meta-analysis of the prevalence of cough
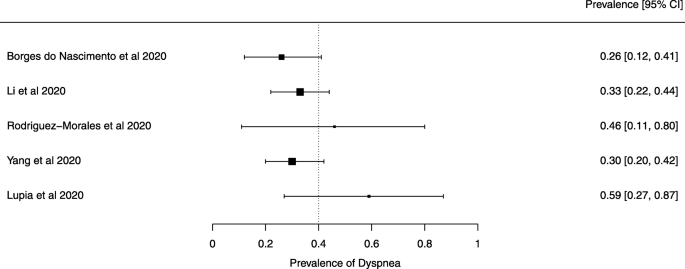
A meta-analysis of the prevalence of dyspnea
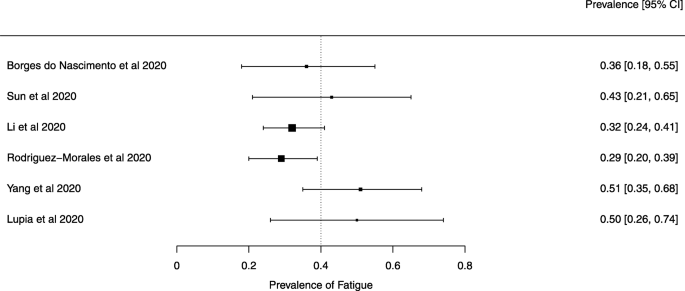
A meta-analysis of the prevalence of fatigue or myalgia
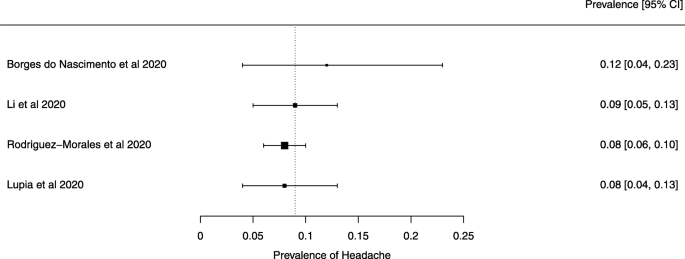
A meta-analysis of the prevalence of headache
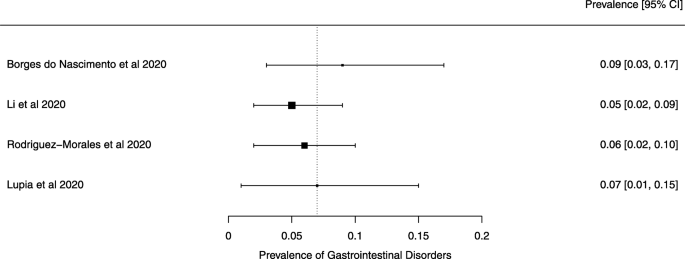
A meta-analysis of the prevalence of gastrointestinal disorders
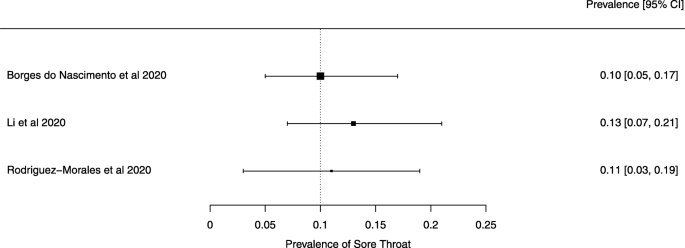
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
SARS-CoV-2 and COVID-19: revisiting the most important research questions
Affiliations.
- 1 School of Biomedical Sciences, The University of Hong Kong, 3/F Laboratory Block, 21 Sassoon Road, Pokfulam, Hong Kong.
- 2 School of Medical and Health Sciences, Tung Wah College, Kowloon, Hong Kong.
- 3 Department of Microbiology, The University of Hong Kong, Pokfulam, Hong Kong.
- 4 School of Biomedical Sciences, The University of Hong Kong, 3/F Laboratory Block, 21 Sassoon Road, Pokfulam, Hong Kong. [email protected].
- PMID: 34922626
- PMCID: PMC8683810
- DOI: 10.1186/s13578-021-00730-1
In February 2020, we highlighted the top nine important research questions on SARS-CoV-2 and COVID-19 concerning virus transmission, asymptomatic and presymptomatic virus shedding, diagnosis, treatment, vaccine development, origin of virus and viral pathogenesis. These and related questions are revisited at the end of 2021 to shed light on the roadmap of bringing an end to the pandemic.
Keywords: Antivirals; COVID-19; Endemic; Pandemic; SARS-CoV-2; Vaccines.
© 2021. The Author(s).
Grants and funding
- C7142-20GF/Research Grants Council, University Grants Committee
- T11-709/21-N/Research Grants Council, University Grants Committee
- COVID190114/Health and Medical Research Fund

IMAGES
VIDEO
COMMENTS
Identifying and prioritizing key research questions is necessary for the rapid development of successful diagnostics, therapeutics, and public health interventions in the fight against COVID-19. The Infectious Diseases Society of America (IDSA) has compiled a comprehensive list of COVID-19 clinical trial and research recommendations for federal ...
Although there have been opportunities for innovation and some potential shifts in expectations, increased caregiving demands associated with the COVID-19 pandemic in 2020, such as remote working, school closures, and childcare and eldercare, had disproportionately negative outcomes for women.
This work reviews and develops some suggested models for the COVID-19 that can address important questions about global health care and suggest important notes. Then, we suggest an updated model that includes a system of differential equations with transmission parameters.
Debates have raged on social media, around dinner tables, on TV, and in Congress about the science of COVID-19. Is it really worse than the flu? How necessary are lockdowns? Do masks work to prevent infection? What kinds of masks work best? Is the new vaccine safe?
COVID-19 has a significant impact on mental health. It is creating a destructive mentality and increasing depression in regular life. As a result, suicidal attempts are increasing. Life of people are becoming more irritable, and people have social anxiety. For COVID-19 social distance has increased. So, physical contact is decreasing.
Here we highlight nine most important research questions concerning virus transmission, asymptomatic and presymptomatic virus shedding, diagnosis, treatment, vaccine development, origin of virus and viral pathogenesis.
In this paper, we fuses multi-source heterogeneous data, and makes predictions for the current COVID-19 epidemic based on the fusion data set, and quantitatively analyzes the model to obtain the quantitative relationship between various factors and the spread of the epidemic.
We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic. Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to ...
The COVID-19 crisis has provided an exceptional opportunity to question the way in which clinical research is conducted, not just for the treatment of COVID-19 patients but also for all other diseases.
In February 2020, we highlighted the top nine important research questions on SARS-CoV-2 and COVID-19 concerning virus transmission, asymptomatic and presymptomatic virus shedding, diagnosis, treatment, vaccine development, origin of virus and viral pathogenesis.