- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

You are here
Nih clinical research trials and you, guiding principles for ethical research.
Pursuing Potential Research Participants Protections

“When people are invited to participate in research, there is a strong belief that it should be their choice based on their understanding of what the study is about, and what the risks and benefits of the study are,” said Dr. Christine Grady, chief of the NIH Clinical Center Department of Bioethics, to Clinical Center Radio in a podcast.
Clinical research advances the understanding of science and promotes human health. However, it is important to remember the individuals who volunteer to participate in research. There are precautions researchers can take – in the planning, implementation and follow-up of studies – to protect these participants in research. Ethical guidelines are established for clinical research to protect patient volunteers and to preserve the integrity of the science.
NIH Clinical Center researchers published seven main principles to guide the conduct of ethical research:
Social and clinical value
Scientific validity, fair subject selection, favorable risk-benefit ratio, independent review, informed consent.
- Respect for potential and enrolled subjects
Every research study is designed to answer a specific question. The answer should be important enough to justify asking people to accept some risk or inconvenience for others. In other words, answers to the research question should contribute to scientific understanding of health or improve our ways of preventing, treating, or caring for people with a given disease to justify exposing participants to the risk and burden of research.
A study should be designed in a way that will get an understandable answer to the important research question. This includes considering whether the question asked is answerable, whether the research methods are valid and feasible, and whether the study is designed with accepted principles, clear methods, and reliable practices. Invalid research is unethical because it is a waste of resources and exposes people to risk for no purpose
The primary basis for recruiting participants should be the scientific goals of the study — not vulnerability, privilege, or other unrelated factors. Participants who accept the risks of research should be in a position to enjoy its benefits. Specific groups of participants (for example, women or children) should not be excluded from the research opportunities without a good scientific reason or a particular susceptibility to risk.
Uncertainty about the degree of risks and benefits associated with a clinical research study is inherent. Research risks may be trivial or serious, transient or long-term. Risks can be physical, psychological, economic, or social. Everything should be done to minimize the risks and inconvenience to research participants to maximize the potential benefits, and to determine that the potential benefits are proportionate to, or outweigh, the risks.
To minimize potential conflicts of interest and make sure a study is ethically acceptable before it starts, an independent review panel should review the proposal and ask important questions, including: Are those conducting the trial sufficiently free of bias? Is the study doing all it can to protect research participants? Has the trial been ethically designed and is the risk–benefit ratio favorable? The panel also monitors a study while it is ongoing.
Potential participants should make their own decision about whether they want to participate or continue participating in research. This is done through a process of informed consent in which individuals (1) are accurately informed of the purpose, methods, risks, benefits, and alternatives to the research, (2) understand this information and how it relates to their own clinical situation or interests, and (3) make a voluntary decision about whether to participate.
Respect for potential and enrolled participants
Individuals should be treated with respect from the time they are approached for possible participation — even if they refuse enrollment in a study — throughout their participation and after their participation ends. This includes:
- respecting their privacy and keeping their private information confidential
- respecting their right to change their mind, to decide that the research does not match their interests, and to withdraw without a penalty
- informing them of new information that might emerge in the course of research, which might change their assessment of the risks and benefits of participating
- monitoring their welfare and, if they experience adverse reactions, unexpected effects, or changes in clinical status, ensuring appropriate treatment and, when necessary, removal from the study
- informing them about what was learned from the research
More information on these seven guiding principles and on bioethics in general
This page last reviewed on March 16, 2016
Connect with Us
- More Social Media from NIH
- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Publications /
A practical guide for health researchers


An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

NIH and Other Federal Guidelines & Policies for Clinical Research
Nih policies & guidelines and other federal regulations for clinical research.
The NIH and other Federal agencies have developed policies, regulations, and guidelines for investigators to follow for conducting safe, ethical, and high-quality clinical research. This page provides information that includes but is not limited to federal and NIH human subjects research policies and guidelines for monitoring clinical research, education and training for investigators, and privacy and protecting confidentiality. For further guidance or questions, reach out to the NIAMS Clinical Management Team at [email protected] .
NIH Human Subjects Policy and Guidance
Policies and guidelines for monitoring clinical research.
- Human Subjects Education, Training, and Resources for Investigators Conducting Clinical Research
- Protecting Privacy and Confidentiality
- Office for Human Research Protections and General Human Subjects Guidelines
U.S. Food and Drug Administration (FDA) Guidelines for Conduct of Clinical Trials
- Gene Therapy, Stem Cells and Fetal Tissue
The NIH has policies that govern the conduct of studies that involve human subjects. We encourage you to review the following guidelines for human subjects research and policies for inclusion of women, children, and individuals across the lifespan in studies. Additionally, this section contains information about single Institutional Review Board (sIRB) and requirements for registering clinical trials on ClinicalTrials.gov.
- NIH Human Subjects Research Policies
- NIH Listing of Select Human Subjects Policy Statement Notices
- NIH Clinical Research Policy
- Removal of the Requirement for IRB Review of NIH Grant Applications Contract
- NIH Policy on the Dissemination of NIH-Funded Clinical Trial Information
- Requirements for Registering Clinical Trials into ClinicalTrials.gov
- Steps to Compliance for NIH awardees
- NIH Grant Application and Proposal Considerations for Human Subjects Research
- Human Subjects System (HSS)
- Annotated Forms Set for NIH Grant Applications-FORMS-F-Series (Human Subjects on Page 32)
- NIH Inclusion Across the Lifespan Policy
- NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research
- Single IRB (sIRB) Policy for Multi-site Research
- Frequently Asked Questions (FAQs), General Questions about Human Subjects
Review the NIH and other federal agency policies for data and safety monitoring in the conduct of clinical trials to ensure the safety of research participants and the appropriate and ethical conduct of the study. Learn the NIAMS requirements and guidelines for reportable events as well as reviewing and reporting unanticipated problems involving risks to human subjects or others and adverse events.
- NIH Policy for Data and Safety Monitoring – June 1998
- Further Guidance on Data and Safety Monitoring for Phase I and II Clinical Trials – June 2000
- NIAMS Data and Safety Monitoring Guidelines and Policies
- NIAMS Safety Reporting Assessment Flowchart
- Guidance on Reporting Incidents to Office for Human Research Protections
- FDA Guidance for Clinical Trial Data Monitoring Committees – March 2006
Human Subjects Education, Training and Resources for Investigators Conducting Clinical Research
NIH investigators and those involved with conducting NIH supported clinical research are expected to be trained and maintain up to date certification on human subjects protection education and good clinical practice (GCP). Here are some useful resources that investigators can refer to which will help them understand the education and training requirements and offer resources to gain knowledge in the various topics related to the safe and ethical conduct of human subjects research.
- Policy on Good Clinical Practice Training for NIH Awardees Involved in NIH-funded Clinical Trials
- NIH Human Subjects Protections Training & Resources
- Training Resources in the Responsible Conduct of Research (RCR) – HHS ORI
- CITI Program Training & Resources
- National Institute of Allergy and Infectious Diseases (NIAID) GCP Learning Center
- National Drug Abuse Treatment Clinical Trials Network (NDAT CTN) GCP Course
- Society of Behavioral Medicine GCP Training for Social and Behavioral Research
- NIH Frequently Asked Questions (FAQs) on Human Subjects Education
Privacy and Confidentiality
Learn more about the policies and guidance for ensuring the confidentiality of individuals who participate in clinical research studies.
- The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule
- HIPAA Administrative Simplification Statute and Rules
- Impact of the HIPAA Privacy Rule on NIH Processes
- NIH Certificates of Confidentiality (CoC) - Human Subjects
OHRP and General Human Subjects Regulations
Learn the procedures investigators must follow in order to protect human subjects who participate in clinical research studies.
- Title 45 Code of Federal Regulations Part 46 – Protection of Human Subjects
- 2020 Edition of International Compilation of Human Research Standards
- OHRP Policy and Guidance Index
- Belmont Report 1979 – Ethical Principles and Guidelines for the Protection of Human Subjects of Research
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Regulatory Guidance
- ICH Guidance for Industry: E6 (R2) Good Clinical Practice
Understand the FDA’s policies and guidance for the conduct of clinical trials as they relate to drugs, devices, and biologics.
- Title 21 Code of Federal Regulations – Food and Drugs
- FDA Clinical Trial Guidance Documents Directory
- Information for Clinical Investigators-Drugs (CDER)
- Information for Clinical Investigators-Devices (CDRH)
- Information for Clinical Investigators-Biologic (CBER)
- FDA Encourages More Participation, Diversity in Clinical Trials
- Notice to NIH Grantees Regarding Letters or Notices from the FDA
Additional Resources:
- Collection of Race and Ethnicity Data in Clinical Trials
- Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Product
- Investigational New Drug Applications (INDs) - Determining Whether Human Research Studies Can Be Conducted Without an IND
- Financial Disclosure by Clinical Investigators
- IRB Responsibilities for Reviewing the Qualifications of Investigators, Adequacy of Research Sites, and the Determination of Whether an IND/IDE is Needed
- FDA and OHRP Final Guidance: Use of Electronic Informed Consent & Questions and Answers
- Elaboration of Definitions of Responsible Party and Applicable Clinical Trial
Gene Therapy, Stem Cells, and Fetal Tissue
Learn the policies and guidelines for conducting clinical research studies that involve gene therapy, stem cells, or fetal tissue.
- NIH Stem Cell Research
- NIH Biosafety, Biosecurity and Emerging Biotechnology
- New Initiatives to Protect Participants in Gene Therapy Trials
- NIH Biosafety Guidelines
- Approval Process for the Use of Human Pluripotent Stem Cells in NIH-Supported Research
- Informed Consent on Use of Human Fetal Tissue
- Changes to Requirements on Human Fetal Tissue Research
- Research on Dried Blood Spots Obtained Through Newborn Screening
- Users' Guide to the Medical Literature
Explore the foundations of evidence-based medicine with JAMA’s Users’ Guide to the Medical Literature collection. Learn to understand and interpret clinical research!
Publication
Article type.
This Users’ Guide to the Medical Literature describes the fundamental concepts of platform trials and master protocols and reviews issues in the conduct and interpretation of these studies.
This Users’ Guide to the Medical Literature provides suggestions for understanding guideline methods and recommendations for clinicians seeking direction in evaluating clinical practice guidelines for potential use in their practice.
- Evaluating Machine Learning Articles JAMA Opinion November 12, 2019 Artificial Intelligence Full Text | pdf link PDF
This Users’ Guide to the Medical Literature discusses the use of machine learning models as a diagnostic tool, and it explains the important steps needed for making these models and the outcomes they derive clinically effective.
This Users’ Guide to the Medical Literature discusses discrimination and calibration, 2 primary ways to measure and compare the accuracy of clinical risk prediction models.
This Users’ Guide to the Medical Literature discusses strategies for adjusting analyses as a way of addressing prognostic imbalance in studies of therapy and harm.
- How to Read a Systematic Review and Meta-analysis and Apply the Results to Patient Care: Users’ Guides to the Medical Literature JAMA Review July 9, 2014 Surgery Ischemic Heart Disease Perioperative Care and Consultation Acute Coronary Syndromes Cardiology Full Text | pdf link PDF has multimedia
Sun and coauthors provide 5 criteria to help clinicians distinguish credible subgroup analyses from spurious subgroup analyses.
- How to Use an Article About Quality Improvement JAMA Review November 24, 2010 Health Care Quality Full Text | pdf link PDF
- How to Use an Article About Genetic Association: C: What Are the Results and Will They Help Me in Caring for My Patients? JAMA Review January 21, 2009 Genetics and Genomics Full Text | pdf link PDF
- How to Use an Article About Genetic Association: B: Are the Results of the Study Valid? JAMA Review January 14, 2009 Genetics and Genomics Full Text | pdf link PDF
- How to Use an Article About Genetic Association: A: Background Concepts JAMA Review January 7, 2009 Genetics and Genomics Full Text | pdf link PDF
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Science & Research
- Science and Research Special Topics
- Clinical Trials and Human Subject Protection
Clinical Trials Guidance Documents
Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice and human subject protection.
Guidance documents are not binding for FDA or the public. Guidance should be viewed as recommendations unless specific regulatory or statutory requirements are cited. An alternative approach may be used if the approach satisfies the requirements of the applicable statute and regulations.
Some links embedded within guidance documents may have changed since the document was published. If a link does not work, please search for the document by title or contact FDA for assistance.
Withdrawn or Expired Clinical Trial Guidance Documents
| Guidance Title | Topic | Draft or Final | Date Issued |
|---|---|---|---|
| Clinical Trials, Administrative / Procedural | Draft | 6/05/2024 | |
| Clinical Trials, Clinical - Medical | Draft | 4/25/2024 | |
| Clinical Trials, Clinical - Medical | Draft | 4/25/2024 | |
| Clinical Trials, Clinical - Medical | Draft | 4/25/2024 | |
| Good Clinical Practice (GCP) | Final | 8/15/2023 | |
| Clinical - Medical | Draft | 5/3/2023 | |
| Administrative / Procedural | Draft | 3/15/2023 | |
| Real World Data/Real World Evidence (RWD/RWE) | Draft | 01/31/2023 | |
| Disqualification, Good Clinical Practice (GCP), Human Subject Protection (HSP), Investigator, Inspection | Final | 12/01/2022 | |
| Clinical - Medical | Final | 10/17/2022 | |
| Clinical - Medical | Draft | 10/17/2022 | |
| Clinical - Medical | Draft | 10/17/2022 | |
| Clinical - Medical | Draft | 09/23/2022 | |
| Real World Data/Real World Evidence (RWD/RWE) Administrative/Procedural | Final | 09/08/2022 | |
| Clinical - Medical | Draft | 12/22/2021 | |
| Device & Drug Safety | Draft | 09/29/2021 | |
| Drug Safety | Draft | 06/25/2021 | |
| Good Clinical Practice (GCP), Human Subject Protection (HSP), Investigator, 1572 | Draft | 05/19/2021 | |
| HIPAA, Human Subject Protection (HSP), Investigation | Final | 11/16/2020 | |
| Design, Diversity, Ethics Committee (EC), Ethnicity, Gender, Good Clinical Practice (GCP), Human Subject Protection (HSP) | Final | 11/10/2020 | |
| Civil Money Penalties, clinicaltrials.gov | Final | 08/14/2020 | |
| Design, Investigation, Labeling, Lactation, Pregnancy, Sponsor | Draft | 07/29/2020 | |
| Coronavirus, COVID, Good Clinical Practice (GCP), Human Subject Protection (HSP), Informed Consent, Institutional Review Board (IRB), Investigation | Final | 07/02/2020 | |
| Electronic, Investigational New Drug (IND), Part 11, Records | Final | 02/21/2020 | |
| Design, Good Clinical Practice (GCP), Investigation, Sponsor | Draft | 12/20/2019 | |
| Clinical — Medical, Design, Good Clinical Practice (GCP), Investigation, Sponsor | Final | 12/02/2019 | |
| Bioavailability, design, drug safety, good clinical practice, investigation, postmarketing | Draft | 10/25/2019 | |
| Exemption, Humanitarian Device Exemption (HDE), Humanitarian Use Device (HUD), Medical Device | Final | 09/06/2019 | |
| Exemption, Humanitarian Device Exemption (HDE), Humanitarian Use Device (HUD), Medical Device | Final | 09/05/2019 | |
| Ethics Committee (EC), Good Clinical Practice (GCP), Human Subject Protection (HSP), Institutional Review Board (IRB), International Conference on Harmonization (ICH), Records, Sponsor | Draft | 08/01/2019 | |
| Design, Investigation, Lactation, Sponsor | Draft | 05/09/2019 | |
| Good Clinical Practice (GCP) | Draft | 03/15/2019 | |
| Clinical, Design, Efficacy, Variability, Protocol, Exclusion, Inclusion, Genomic | Final | 03/15/2019 | |
| Children, Human Subject Protection (HSP), Informed Consent, Institutional Review Board (IRB), Pediatric | Final | 03/13/2019 | |
| Common Rule, Human Subject Protection (HSP), Institutional Review Board (IRB) | Final | 10/11/2018 | |
| Design, Ethics Committee (EC), Good Clinical Practice (GCP), Human Subject Protection (HSP), Institutional Review Board (IRB), International Conference on Harmonization (ICH), Records, Sponsor | Final | 07/19/2018 | |
| Institutional Review Board (IRB), Records | Final | 05/17/2018 | |
| Children, Ethics Committee (EC), Good Clinical Practice (GCP), Human Subject Protection (HSP), Institutional Review Board (IRB), International Conference on Harmonization (ICH), Records, Sponsor | Final | 04/11/2018 | |
| Gender, Human Subject Protection (HSP), Institutional Review Board (IRB), Investigation, Pregnancy | Draft | 04/09/2018 | |
| Ethics Committee (EC), Good Clinical Practice (GCP), Human Subject Protection (HSP), Informed Consent, Institutional Review Board (IRB), Sponsor | Final | 03/01/2018 | |
| Good Clinical Practice (GCP), Investigation, Investigational Device Exemption (IDE), Medical Device | Final | 02/21/2018 | |
| Human Subject Protection (HSP), Informed Consent | Final | 01/29/2018 | |
| Good Clinical Practice (GCP), In Vitro Diagnostic (IVD), Investigation, Investigational Device Exemption (IDE), Investigational New Drug (IND), Labeling, Medical Device, Sponsor | Draft | 12/18/2017 | |
| Human Subject Protection (HSP), Institutional Review Board (IRB), Waiver | Final | 10/03/2017 | |
| Human Subject Protection (HSP), Informed Consent, Institutional Review Board (IRB), Investigation, Investigational New Drug (IND) | Final | 10/03/2017 | |
| Institutional Review Board (IRB), Records | Final | 09/25/2017 | |
| Demographic, Diversity, Ethnicity, Gender, Investigation, Medical Device, Pediatric | Final | 09/12/2017 | |
| Good Clinical Practice (GCP), Human Subject Protection (HSP), Electronic, Investigation, Part 11, Records | Draft | 06/21/2017 | |
| Certify, Certification, clinicaltrials.gov, Medical Device | Final | 06/07/2017 | |
| Emergency, Human Subject Protection (HSP), Informed Consent, Institutional Review Board (IRB), Investigation, Investigational New Drug (IND) | Final | 01/13/2017 | |
| Electronic, Good Clinical Practice (GCP), Human Subject Protection (HSP), Informed Consent, Part 11, Records | Final | 12/15/2016 | |
| Advisory Committees, Clinical — Medical, Good Clinical Practice (GCP), Humanitarian Device Exemption (HDE), Humanitarian Use Device (HUD), Labeling, Laser Notice, Medical Device, Neurological, Premarket, Premarket Approval (PMA), Safety, Sponsor | Final | 11/07/2016 | |
| Demographic, Ethnicity, Race | Final | 10/26/2016 | |
| Good Clinical Practice (GCP), Humanitarian Device Exemption (HDE), Humanitarian Use Device (HUD), Investigational Device Exemption (IDE), Medical Device, Premarket, Premarket Approval (PMA), Sponsor, 510k | Final | 07/27/2016 | |
| Chemistry Manufacturing Controls (CMC), Good Clinical Practice (GCP), Investigation, Sponsor | Final | 06/30/2016 | |
| Children, Human Subject Protection (HSP), Medical Device | Final | 06/21/2016 | |
| Good Clinical Practice (GCP), Investigation, Investigational New Drug (IND) | Final | 06/03/2016 | |
| Human Subject Protection (HSP), Informed Consent, Institutional Review Board (IRB), Investigation, Investigational New Drug (IND) | Final | 06/03/2016 | |
| Good Clinical Practice (GCP), Humanitarian Device Exemption (HDE), Humanitarian Use Device (HUD), Investigational Device Exemption (IDE), Medical Device, Premarket, Premarket Approval (PMA), Sponsor, 510k | Final | 03/07/2016 | |
| Design, Investigation, Labeling, Lactation, Pregnancy, Sponsor | Final | 06/10/2015 | |
| Human Subject Protection (HSP), Informed Consent, Institutional Review Board (IRB) | Draft | 09/15/2014 | |
| Demographic, Design, Gender, Good Clinical Practice (GCP), Medical Device, Premarket, Sponsor | Final | 08/22/2014 | |
| Institutional Review Board (IRB) | Final | 05/23/2014 | |
| Informed Consent, Institutional Review Board (IRB) | Final | 03/31/2014 | |
| In Vitro, Investigation, Medical Device, Specimen | Final | 11/25/2013 | |
| Design, Good Clinical Practice (GCP), Investigation, Medical Device, Premarket, Sponsor | Final | 11/07/2013 | |
| Institutional Review Board (IRB), Investigation, Medical Device, Safety | Final | 10/01/2013 | |
| Good Clinical Practice (GCP), Electronic, Part 11, Records | Final | 09/18/2013 | |
| Institutional Review Board (IRB), Investigation, Investigational New Drug (IND) | Final | 09/10/2013 | |
| Institutional Review Board (IRB), Investigational Device Exemption (IDE), Investigational New Drug (IND), Investigator, Medical Device | Final | 08/27/2013 | |
| Good Clinical Practice (GCP) | Final | 08/07/2013 | |
| Good Clinical Practice (GCP), Human Subject Protection (HSP), Informed Consent, Institutional Review Board (IRB) | Final | 04/01/2013 | |
| Financial Disclosure, Good Clinical Practice (GCP) | Final | 02/01/2013 | |
| Good Clinical Practice (GCP), International Conference on Harmonization (ICH), Sponsor | Final | 01/29/2013 | |
| Bioavailability, Bioequivalence, Good Clinical Practice (GCP), Investigation, Investigational New Drug (IND), Safety | Final | 12/20/2012 | |
| Bioavailability, Bioequivalence, Good Clinical Practice (GCP), Investigation, Investigational New Drug (IND), Safety | Final | 12/20/2012 | |
| Good Clinical Practice (GCP), Investigation, Investigational New Drug (IND) | Final | 03/01/2012 | |
| Design, Diversity, Ethnicity, Gender, International Conference on Harmonization (ICH), Sponsor | Final | 03/01/2012 | |
| Continuing Review, Institutional Review Board (IRB) | Final | 02/27/2012 | |
| Human Subject Protection (HSP), Informed Consent, Institutional Review Board (IRB) | Final | 02/01/2012 | |
| Good Clinical Practice (GCP), Human Subject Protection (HSP), Informed Consent | Draft | 08/19/2011 | |
| Institutional Review Board (IRB), Investigational New Drug (IND) | Final | 08/02/2010 | |
| Human Subject Protection (HSP), In Vitro, Informed Consent, Medical Device, Specimen | Final | 06/25/2010 | |
| Good Clinical Practice (GCP), Human Subject Protection (HSP), Investigator, 1572 | Final | 06/04/2010 | |
| Good Clinical Practice (GCP), Human Subject Protection (HSP), Inspection, Investigator | Final | 06/01/2010 | |
| Investigation, Investigational New Drug (IND), Product Development | Final | 12/09/2009 | |
| Clinical Investigator, Institutional Review Board (IRB) | Final | 10/23/2009 | |
| Institutional Review Board (IRB), Registration | Final | 07/09/2009 | |
| Institutional Review Board (IRB), Report, Safety | Final | 01/14/2009 | |
| Drug, Labeling, Practice, Medical Device, Medicine, Sponsor | Final | 12/31/2008 | |
| Good Clinical Practice (GCP), Human Subject Protection (HSP), Informed Consent | Final | 10/01/2008 | |
| Good Clinical Practice (GCP), Good Manufacturing Practice (GMP), Investigation | Final | 07/14/2008 | |
| In Vitro Diagnostic (IVD), Laboratory Developed Test (LDT), Medical Device, Reagent | Final | 09/13/2007 | |
| Good Clinical Practice (GCP), Electronic, Part 11, Records | Final | 05/10/2007 | |
| Children, Human Subject Protection (HSP), Informed Consent, Institutional Review BA/BEoard (IRB), Pediatric | Final | 12/01/2006 | |
| Design, Diversity, Ethics Committee (EC), Ethnicity, Gender, Human Subject Protection (HSP), Institutional Review Board (IRB), International Conference on Harmonization (ICH), Sponsor | Final | 09/01/2006 | |
| Human Subject Protection (HSP), In Vitro, Informed Consent, Medical Device, Specimen | Final | 04/25/2006 | |
| Good Clinical Practice (GCP), Human Subject Protection (HSP), Institutional Review Board (IRB) | Final | 03/28/2006 | |
| Institutional Review Board (IRB), Multi—center, Single | Final | 03/16/2006 | |
| Investigation, Labeling, Sponsor | Final | 01/24/2006 | |
| Good Clinical Practice (GCP), Investigation, Investigational New Drug (IND) | Final | 01/12/2006 | |
| Institutional Review Board (IRB), Medical Device, Risk, Safety | Final | 01/01/2006 | |
| Institutional Review Board (IRB), Medical Device, Risk, Safety | Final | 01/01/2006 | |
| Human Subject Protection (HSP), Institutional Review Board (IRB), Inspection | Final | 01/01/2006 | |
| Good Clinical Practice (GCP), Investigational New Drug (IND), Risk, Safety | Final | 03/29/2005 | |
| Informed Consent, Risk, Safety, Sponsor | Final | 03/29/2005 | |
| Clinical — Medical, Good Clinical Practice (GCP) | Final | 03/24/2005 | |
| Pharmacogenomic, Product Development | Final | 03/23/2005 | |
| Good Clinical Practice (GCP), Hold, Investigation, Investigational New Drug (IND), Investigator, Misconduct | Final | 09/02/2004 | |
| Biotechnology, Clinical Trial, Protocol | Final | 08/19/2004 | |
| Bioavailability, Bioequivalence | Final | 05/25/2004 | |
| Financial Disclosure, Good Clinical Practice (GCP), Institutional Review Board (IRB) | Final | 05/05/2004 | |
| Exemption, Good Clinical Practice (GCP), Investigation, Investigational New Drug (IND) | Final | 01/15/2004 | |
| Electronic, Good Clinical Practice (GCP), Part 11, Records | Final | 09/05/2003 | |
| HIPAA, Institutional Review Board (IRB) | Final | 08/16/2003 | |
| Chemistry Manufacturing Controls (CMC), Investigation, Investigational New Drug (IND), Sponsor | Final | 05/20/2003 | |
| Bioavailability, Bioequivalence, Drug, Food, Investigational New Drug (IND) | Final | 12/01/2002 | |
| Electronic, Investigation, Medical Device, Part 11, Quality, Risk, Software, Validation | Final | 01/11/2002 | |
| Design, Ethics Committee (EC), Good Clinical Practice (GCP), Human Subject Protection (HSP), Institutional Review Board (IRB), International Conference on Harmonization (ICH), Records, Sponsor | Final | 05/14/2001 | |
| Children, Ethics Committee (EC), Good Clinical Practice (GCP), Human Subject Protection (HSP), Institutional Review Board (IRB), International Conference on Harmonization (ICH), Records, Sponsor | Final | 12/15/2000 | |
| Good Clinical Practice (GCP), Hold, Investigation, Investigational New Drug (IND), Investigator | Final | 10/01/2000 | |
| Expedited Review, Good Clinical Practice (GCP), Human Subject Protection (HSP), Institutional Review Board (IRB) | Final | 11/09/1998 | |
| Design, Diversity, Ethics Committee (EC), Ethnicity, Gender, International Conference on Harmonization (ICH), Sponsor | Final | 06/10/1998 | |
| Demographic, Gender, Good Clinical Practice (GCP), Human Subject Protection (HSP), Investigation, Investigational New Drug (IND) | Final | 01/01/1998 | |
| Human Subject Protection (HSP), Institutional Review Board (IRB) | Final | 01/01/1998 | |
| Institutional Review Board (IRB) | Final | 01/01/1998 | |
| Institutional Review Board (IRB) | Final | 01/01/1998 | |
| Good Clinical Practice (GCP), Institutional Review Board (IRB), Investigator, Sponsor | Final | 01/01/1998 | |
| Human Subject Protection (HSP), Informed Consent, Institutional Review Board (IRB), Recruit, Recruitment | Final | 01/01/1998 | |
| Human Subject Protection (HSP), Informed Consent | Final | 01/01/1998 | |
| Human Subject Protection (HSP), Informed Consent, Recruit, Recruitment | Final | 01/01/1998 | |
| Human Subject Protection (HSP), Informed Consent, Investigation, Investigational Device Exemption (IDE), Investigational New Drug (IND), Institutional Review Board (IRB), Medical Device | Final | 01/01/1998 | |
| Investigation, Investigational New Drug (IND), Medical Device, Practice of Medicine | Final | 01/01/1998 | |
| Emergency, Human Subject Protection (HSP), Informed Consent, Investigational New Drug (IND), Institutional Review Board (IRB) | Final | 01/01/1998 | |
| Ethics Committee (EC), Good Clinical Practice (GCP), Human Subject Protection (HSP), Institutional Review Board (IRB), International Conference on Harmonization (ICH), Records, Sponsor | Final | 12/17/1997 | |
| Design, Good Manufacturing Practice (GMP), Medical Device, Sponsor | Final | 03/11/1997 | |
| Good Clinical Practice (GCP), International Conference on Harmonization (ICH), Sponsor | Final | 07/01/1996 | |
| Ethics Committee (EC), Design, Diversity, Ethnicity, Gender, Human Subject Protection (HSP), International Conference on Harmonization (ICH), Sponsor | Final | 08/01/1994 | |
| Demographic, Gender, Good Clinical Practice (GCP), Human Subject Protection (HSP), Investigation, Investigational New Drug (IND) | Final | 07/22/1993 |
Resources For You
- Electronic Code of Federal Regulations (eCFR)
- Search for FDA Guidance
- Websites with Information about Clinical Trials
Clinical Practice: Home
- Drug Information
- Related Research Guides
References for a Quick Lookup
These core titles are available both online and in print.
- Stat-Ref Stat!Ref is a web-based platform that aggregates core health sciences books (textbooks, handbooks, drug books, etc.) from leading publishers. It enables you to cross search medical textbooks and resources: also gives access to the full-text of these books through the table of contents. Other content include Stedman's Medial Dictionary and MedCalc, a collection of medical algorithms, risk prediction tools, and other handy clinical calculation tools.
Most Popular Databases
These popular databases allow you to search for journal articles by subject
- PubMed with full text This link will take you to PubMed through the Harvard proxy server. This will allow you easy access to the full text of journals with a Harvard subscription. When you open an article abstract you will see the Find It@Harvard icon on the upper right. Click on this icon and you should either see the full text article or Harvard's print holdings in the HOLLIS catalog. more... less... Find it at Harvard
- EMBASE EMBASE is a European biomedical database. It indexes approximately 1,500 biomedical journals that are not indexed in PubMed. EMBASE excels in indexing drug information. more... less... EMBASE is a biomedical and pharmacological database containing abstracts and citations from 1974 to the present. The records reflect all current developments in biomedical and drug-related fields. The EMBASE journal collection is international in scope, covering subjects including Drug Research, Pharmacology, Pharmacy, Pharmacoeconomics, Pharmaceutics and Toxicology, Human Medicine (Clinical and Experimental), Basic Biological Research, Health Policy and Management, Public, Occupational and Environmental Health, Substance Dependence and Abuse, Psychiatry, Forensic Science and Biomedical Engineering and Instrumentation
- CINAHL Plus with Full Text (EBSCO) CINAHL provides indexing for close to 3000 journals from the fields of nursing and allied health. The database contains more than 1,000,000 records dating back to 1981. This database offers access to health care books, nursing dissertations, selected conference proceedings, standards of practice, educational software, audiovisuals and book chapters. Full text material includes many journals plus legal cases, clinical innovations, critical paths, drug records, research instruments and clinical trials. more... less... CINAHL Plus with Full Text is a comprehensive source of full text for nursing & allied health journals, providing full text for more than 560 journals indexed in CINAHL.
- The Cochrane Library Consists of 4 databases: Cochrane database of systematic reviews (CDSR); Database of abstracts of reviews of effectiveness (DARE); Cochrane controlled trials register (CCTR); and Cochrane review methodology database (CRMD)
- Health & Psychosocial Instruments - HAPI (EBSCOhost) A database designed to help users identify measurement tests used in health, psychosocial sciences, organizational behavior, and library and information science; provides source, abstract, and reviewer(s) when applicable. more... less... Health and Psychosocial Instruments (HaPI), produced by Behavioral Measurement Database Services, is a comprehensive bibliographic database providing information about behavior measurement instruments. Information in the database is abstracted from hundreds of leading journals covering health sciences and psychosocial sciences. Additionally, instruments from Industrial/Organizational Behavior and Education are included. Records contained in HaPI provide information on questionnaires, interview schedules, vignettes/scenarios, coding schemes, rating and other scales, checklists, indexes, tests, projective techniques, and more.
Clinical Tools
Point of care quick reference, calculators and decision support tools
- DxPlain Differential Diagnosis tool. Enter symptoms; receive prompts for findings; add more information and receive most likely common and rare diagnoses and lists of recommended tests. more... less... From the MGH Computer lab. eCommons or HU log in required.
- Next: Drug Information >>
- Last Updated: May 6, 2020 3:16 PM
- URL: https://guides.library.harvard.edu/clinical
| | |

Principles of Research Methodology
A Guide for Clinical Investigators
- © 2012
- Phyllis G. Supino 0 ,
- Jeffrey S. Borer 1
, Cardiovascular Medicine, SUNY Downstate Medical Center, Brooklyn, USA
You can also search for this editor in PubMed Google Scholar
, Cardiovascualr Medicine, SUNY Downstate Medical Center, Brooklyn, USA
- Based on a highly regarded and popular lecture series on research methodology
- Comprehensive guide written by experts in the field
- Emphasizes the essentials and fundamentals of research methodologies
76k Accesses
23 Citations
7 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
Principles of Research Methodology: A Guide for Clinical Investigators is the definitive, comprehensive guide to understanding and performing clinical research. Designed for medical students, physicians, basic scientists involved in translational research, and other health professionals, this indispensable reference also addresses the unique challenges and demands of clinical research and offers clear guidance in becoming a more successful member of a medical research team and critical reader of the medical research literature. The book covers the entire research process, beginning with the conception of the research problem to publication of findings. Principles of Research Methodology: A Guide for Clinical Investigators comprehensively and concisely presents concepts in a manner that is relevant and engaging to read. The text combines theory and practical application to familiarize the reader with the logic of research design and hypothesis construction, the importance of research planning, the ethical basis of human subjects research, the basics of writing a clinical research protocol and scientific paper, the logic and techniques of data generation and management, and the fundamentals and implications of various sampling techniques and alternative statistical methodologies. Organized in thirteen easy to read chapters, the text emphasizes the importance of clearly-defined research questions and well-constructed hypothesis (reinforced throughout the various chapters) for informing methods and in guiding data interpretation. Written by prominent medical scientists and methodologists who have extensive personal experience in biomedical investigation and in teaching key aspects of research methodology to medical students, physicians and other health professionals, the authors expertly integrate theory with examples and employ language that is clear and useful for a general medical audience. A major contribution to the methodology literature, Principles of Research Methodology: A Guide for Clinical Investigators is an authoritative resource for all individuals who perform research, plan to perform it, or wish to understand it better.
Similar content being viewed by others

History of Research

Research (See Clinical Research; Research Ethics)

General Study Objectives
Table of contents (13 chapters), front matter, overview of the research process.
Phyllis G. Supino
Developing a Research Problem
- Phyllis G. Supino, Helen Ann Brown Epstein
The Research Hypothesis: Role and Construction
Design and interpretation of observational studies: cohort, case–control, and cross-sectional designs.
- Martin L. Lesser
Fundamental Issues in Evaluating the Impact of Interventions: Sources and Control of Bias
Protocol development and preparation for a clinical trial.
- Joseph A. Franciosa
Data Collection and Management in Clinical Research
- Mario Guralnik
Constructing and Evaluating Self-Report Measures
- Peter L. Flom, Phyllis G. Supino, N. Philip Ross
Selecting and Evaluating Secondary Data: The Role of Systematic Reviews and Meta-analysis
- Lorenzo Paladino, Richard H. Sinert
Sampling Methodology: Implications for Drawing Conclusions from Clinical Research Findings
- Richard C. Zink
Introductory Statistics in Medical Research
- Todd A. Durham, Gary G. Koch, Lisa M. LaVange
Ethical Issues in Clinical Research
- Eli A. Friedman
How to Prepare a Scientific Paper
Jeffrey S. Borer
Back Matter
From the reviews:
Editors and Affiliations
Bibliographic information.
Book Title : Principles of Research Methodology
Book Subtitle : A Guide for Clinical Investigators
Editors : Phyllis G. Supino, Jeffrey S. Borer
DOI : https://doi.org/10.1007/978-1-4614-3360-6
Publisher : Springer New York, NY
eBook Packages : Medicine , Medicine (R0)
Copyright Information : Springer Science+Business Media, LLC 2012
Hardcover ISBN : 978-1-4614-3359-0 Published: 22 June 2012
Softcover ISBN : 978-1-4939-4292-3 Published: 23 August 2016
eBook ISBN : 978-1-4614-3360-6 Published: 22 June 2012
Edition Number : 1
Number of Pages : XVI, 276
Topics : Oncology , Cardiology , Internal Medicine , Endocrinology , Neurology
- Publish with us
Policies and ethics
- Find a journal
- Track your research

Posters advertising a variety of clinical research trials at Penn State College of Medicine are seen on a College bulletin board in summer 2016. The image shows five posters in a line, with the center one in focus and the others out-of-focus in the background.
- Clinical Research Guidebook
See all COVID-19 research updates, including updated human-subjects research guidance and participant screening script, here.
This clinical research guidebook has been developed for faculty and staff members engaged in clinical research at Penn State College of Medicine/Penn State Health Milton S. Hershey Medical Center. It has been adapted from the materials created and released by The Clinical Trials Resource Group at the University of California – Davis CTSC.
Researchers at University Park may wish to view University Park-specific guidebook information .
Request clinical research project help here
Jump to topic
Resources and training.
Penn State Health and Penn State College of Medicine conduct a variety of clinical research studies in accordance with the applicable regulations relevant to the protection of human subjects. For FDA-regulated research, Penn State commits to apply the “International Conference on Harmonisation – Good Clinical Practice as adopted by the U.S. FDA and as required by sponsors. Standardized training and continuing skill development of all clinical research professionals is an important part of preparation for clinical research. It is the responsibility of all staff and investigators to know, understand and maintain sufficient knowledge of the federal, state and local requirements protecting research subjects.
The U.S. Department of Health and Human Services (HHS) is the government’s principal agency for protecting the health of all Americans. It comprises several public health services agencies including the FDA (Food and Drug Administration), OHRP (Office of Human Research Protection), the NIH (National Institutes of Health), and the Centers for Medicare and Medicaid Services (CMS).
Food and Drug Administration (FDA, fda.gov ) is responsible for protecting and promoting public health through the regulations and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), veterinary products, and cosmetics. Understanding these rules is critical for any investigator who conducts human subject studies with drugs, devices or dietary supplements, whether already approved on the market, or still investigational.
Office of Human Research Protection (OHRP, hhs.gov/ohrp ) provides leadership, guidance, and education in the protection of the rights, welfare, and well-being of subjects involved in research conducted or supported by the HHS. OHRP performs these services through providing clarification and guidance, developing educational programs and materials, maintaining regulatory oversight, and providing advice on ethical and regulatory issues in biomedical and social-behavioral research. Detailed regulations for human subject protection are listed on the OHRP website . OHRP rules guide the Institutional Review Boards (IRBs).
National Institutes of Health (NIH, nih.gov ) seeks to provide fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce the burdens of illness and disability. As part of this mission NIH provides leadership and direction to programs designed to improve health and provides support for research.
The NIH funds over 60 Clinical and Translational Science Centers across the country. Working together as a national consortium, Clinical Translational Science Award (CTSA) institutions share a common vision to improve human health by transforming the research and training environment to enhance the efficiency and quality of clinical and translational research. The CTSA program is supported by the National Center for Advancing Translational Science (NCATS), part of the National Institutes of Health.
The CTSA program has the following overriding objectives:
- Provide a comprehensive array of essential tools and services to spark clinical and translational research.
- Ensure the training of a well prepared workforce of trainees, staff, and investigators.
- Effectively communicate the many tools, services, and training opportunities to ensure innovative translational science advances that will improve human health.
Today, Penn State Clinical and Translational Science Institute ( ctsi.psu.edu ) offers resources that faculty, trainees and staff across the scientific and medical spectrum can use to enhance research and improve health and healthcare delivery.
Centers for Medicare and Medicaid Services (CMS, cms.gov ) is the federal agency which administers Medicare, Medicaid, and the Children’s Health Insurance Program. On June 7, 2000, the President of the United States issued an executive memorandum directing the Secretary of Health and Human Services to “explicitly authorize [Medicare] payment for routine patient care costs… and costs due to medical complications associated with participating in clinical trials.” CMS responded to the executive order with the clinical trial policy – National Coverage Determination (NCD). Medicare State fiscal intermediaries also issue Local Coverage Determinations (LCD). Our intermediary is Novitas Solutions, Inc.
Understanding Coverage Rules is critical for generating correct billing claims for clinical research participants. At Penn State Health/Penn State College of Medicine, the tool and the process of applying CMS rules to each individual study is called Coverage Analysis . This information is reviewed in detail in the Preparing Documents section of this guidebook.
The Code of Federal Regulations (CFR) is a compendium of the general and permanent rules and regulations published in the Federal Register by the federal executive departments and agencies. The CFR is divided into 50 titles that represent broad areas subject to Federal regulations. Title 45 CFR encompasses regulation of Public Welfare. Title 21 CFR is administered by the FDA and covers regulations of Food and Drugs.
Title 45 CFR 46 ( The Common Rule ) is a core set of regulations defining protection of Human Subjects in clinical research. 45 CFR part 46 includes four subparts:
- Subpart A , also known as the Federal Policy or the “Common Rule”
- Subpart B , additional protections for pregnant women, human fetuses and neonates
- Subpart C , additional protections for prisoners
- Subpart D , additional protections for children
Through a system of IRB registration and assurances , the Department of Health & Human Services (DHHS) regulations require institutions to commit to compliance with 45 CFR 46 before initiating participation in DHHS-conducted or -supported research involving human subjects. A Federalwide Assurance (FWA) is the institution’s commitment to apply 45 CFR 46 as required. Penn State College of Medicine’s FWA is 00004251 . In the FWA, Penn State Health is listed as a component of Penn State College of Medicine.
Title 21 CFR: The FDA regulations (Title 21 CFRs) are applicable when research is being conducted to develop a medical product that will be licensed for sale in the United States. Certain federally sponsored and privately sponsored research is subject to the regulations of the FDA according to 21 CFR Parts 50 and 56. Title 21 CFR part 50 defines regulations for informed consent and 21 CFR part 56 defines regulations for IRBs. These regulations largely overlap but are not identical with the Common Rule. Investigators need to know both sets of regulations to apply them appropriately.
Title 21 CFR 312 details the regulations for human research done with investigational drugs. This Title includes, but is not limited to, the regulations for applying to FDA to conduct research under an Investigational New Drug (IND) application (21 CFR 312 Subpart B), responsibilities of Sponsors and Investigators under an IND (21 CFR 312 Subpart D), and expanded access to Investigational Drugs (21 CFR 312 Subpart I). The IND and IDE Submissions section of this guidebook discusses the drug development process in more detail.
Title 21 CFR 812 details the regulations for human research with investigational devices. The regulations lay out the framework for applying to FDA to conduct human subjects research with Investigational Devices (21 CFR 812 Subpart B), responsibilities of Sponsors (21 CFR 812 Subpart C) and Investigators (21 CFR 812 Subpart E), and IRB approval 21 CFR 812 Subpart D).
The IND and IDE Submissions section of this guidebook discusses the drug development process in more detail.
This guidebook is updated on an annual basis at minimum to provide updates and new information. Always reference this website, not printouts, for the most recent information.
HRP-103 – Investigator Manual is designed to guide investigators and study team members through policies and procedures related to the conduct of Human Research that are specific to this institution. General information regarding Human Research protections and relevant federal regulations and guidance is incorporated into the required human protections training.
It is recommended that all study team members review the Investigator Manual and become familiar with its contents. The manual is updated regularly and can serve as an initial source of information when questions arise regarding policies and procedures.
The manual can be located from the link below or accessed through the CATS IRB library.
Access the Investigator Manual
Penn State College of Medicine Clinical Trials Office (CTO) creates and maintains multiple Standard Operating Procedures (SOPs) and competencies related to conduct of clinical research at Penn State College of Medicine and Penn State Health.
The SOPs as well as links to other institutional research resources can be found, including coordinator competencies, can be found on the Penn State Health Policy Portal (ePass login required).
Penn State employs the Collaborative Institutional Training Initiative (CITI) program, a web-based training program to satisfy the training requirements for all personnel conducting human subject research as part of the University and/or Penn State Health.
For details on required modules, see IRB training and resources on the University Office of Research Protections website.
Penn State College of Medicine and Penn State Health have partnered with the Association of Clinical Research Professionals (ACRP) to support professional growth and development through providing membership accounts to users registered through the organizational account. User seats are currently capped at 50 members with anticipation to increase capacity based on need and utilization if funding permits. ACRP Membership through the organizational account immediately connects users with:
- 200-plus on-demand training, continuing education and ACRP Certification Exam preparation modules available in a Penn State College of Medicine and Penn State Health-branded learning environment
- Unlimited ACRP contact hours for ACRP certification renewal
- Breaking news and regulatory updates
- ACRP’s community and members-only discussion groups
- Plus, ACRP member pricing for ACRP certification and the ACRP annual conference
Please contact Liz Galgocy at [email protected] with questions, for access instructions or to be added to the waiting list for account access.
The institution provides a number of training opportunities to be sure our workforce members are HIPAA compliant. The courses are designed to satisfy accreditation, contractual and regulatory requirements, and they range from online courses used during New Employee Orientation, to introductory and refresher presentations available to employees through Compass. Cybersecurity and Privacy Annual Training is assigned yearly through Compass and completion is required to maintain compliance for continued employment (login required).
Required trainings include:
- CITI Yearly Biosafety Training
- Safety Annual Training 100
- Safety Annual Training College of Medicine 100
- Biological Shipping and Dry Ice Training
- COM Bloodborne Pathogens Training or Infection Prevention/Control Training
Lab Safety Training or Biological Safety, Chemical/Laboratory Safety, and Hazardous Waste Management and Minimization
Lab safety training is for research laboratory personnel in the Penn State College of Medicine. Every employee working in the research lab is required to take general safety training on an annual basis. Learn more on the Department of Safety section of the Infonet (login required).
Annual blood-borne pathogen training is required for labs currently using unfixed human and or non-human primate materials including human derived cell lines. Learn more on the Department of Safety section of the Infonet (login required).
SAA (Satellite Accumulation Area) training is required for each laboratory to have a representative registered and trained in the hazardous waste disposal and minimization program. Learn more on the Department of Safety section of the Infonet (login required).
CITI Biosafety/Biosecurity training is required by the Principal Investigator if operating a lab on the College of Medicine campus. The training is highly recommended for all laboratory personnel including technicians, technologists, postdoctoral scholars and visiting scientists. See more information at citi.psu.edu .
View more information about researching compliance training requirements
New Submitter Training is conducted by the IRB for submissions to the Centralized Application Tracking System Institutional Review Board (CATS IRB). This orientation provides detailed training on the ethical principles of human research, an explanation of the researcher’s primary responsibility for protecting research subjects and for complying with all applicable provisions of institutional, state and federal laws. It provides an explanation of the different levels of IRB review and describes the processes for IRB submissions. Find upcoming IRB trainings and workshops . The clinical trials management system, Study Tracking and Analysis for Research (STAR) , training is provided by the CTO. Learn more on the STAR Infonet section (login required). There may be additional training requirements based on your departmental requirements.
Information pertaining to Payer Coverage Analysis and clinical trial budgeting is available through the Clinical Trials Office .
All investigators who are engaged in research must complete Penn State University’s required FCOI training and submit a disclosure of significant financial interest. Per PSU Policy RP06, an investigator is defined as: “any individual, regardless of his or her title or position, whether faculty, staff, or student, who has the ability to make independent decisions related to the design, conduct or reporting of University Research, but not including individuals who perform only incidental or isolated tasks related to a University Research project.” Disclosure is required prior to the submission of an application for research funding, at least annually, and within 30 days of the discovery or acquisition of a new Significant Financial Interest. The Disclosure must identify significant financial interests of the investigator, spouses/partners, and dependent children that exceed the thresholds set by PSU and that relate to any of the investigator’s institutional responsibilities. Additionally, the College of Medicine has specific disclosure requirements for financial interest related to either human subjects research or purchasing responsibility. Both FCOI training and disclosure are completed via Penn State University’s electronic Conflict of Interest System, COINS ( coins.psu.edu ). As part of the electronic Disclosure Form, COINS requires investigators to complete FCOI training upon their first disclosure and again every four years. For details, please see the College of Medicine Conflict of Interest Program Overview and PSU Policy RP06 Disclosure and Management of Significant Financial Interests .
There are additional requirements for when a Penn State research project includes research procedures on-site in a Penn State Health or Penn State College of Medicine facility, and the onsite study team includes employees of Penn State who are not specifically a student or employee of the health system or College of Medicine. These requirements are not related to research, and do not apply to study team members that will never be in person at a Penn State Health or College of Medicine facility. These requirements are driven by the health system and requirements from the joint commission, the accrediting body for US health care organizations and programs. The joint commission requires that the exact same standards be applied to “all members of the workforce.” Anyone working on site at Penn State Health or Penn State College of Medicine for 5 or more days is considered a member of the workforce and must complete the same clearances as regular employees on campus.
View information related to the requirements and related guidance when these circumstances exist (in Sharepoint; Penn State Access ID login required).
The Penn State Research Portal (Pure) is a publicly-available system that captures and displays the research output of the University, both for investigators and units, and facilitates collaboration between investigators across the University and beyond. Pure is one of several applications by the company Elsevier. Pure aggregates research information from internal and external sources and enhances the visibility and discoverability of research at Penn State, both internally and externally. It provides detailed information on scholarly output, publications, networks, citation data from journals and social media citations. See details about Pure here .
For further information, resources, and assistance in identifying collaborators and funding opportunities, please also visit the Research Development website .
There are multiple central research administration support offices throughout the organization. Click on each link provided for information regarding each of these offices.
- Center for Medical Innovation (CMI)
- Clinical Trials Office (CTO)
- Human Research Protection Program (HRPP)
- Penn State Clinical and Translational Science Institute (CTSI)
- Office of Research Affairs (ORA)
- Research Development
- Research Quality Assurance (RQA)
Study Development and Feasibility: CTSI Resources
Penn State Clinical and Translational Science Institute (CTSI) can provide a wide range of consultation services during all stages of studies, and specifically during the project development and start-up phases. The new Research Navigator service provides hands-on support in conducting research. See CTSI consultation services and request Research Navigator assistance.
The mission of the College of Medicine Clinical Trials Office is to enhance, foster and promote organized, high-quality clinical research within Penn State Health Milton S. Hershey Medical Center and Penn State College of Medicine.
By promoting clinical research, the Clinical Trials Office helps Penn State Health and Penn State College of Medicine meet its mission goals of excellence in patient care, education, research and community service.
Established in the 1990s, current services offered to support investigators include protocol and budget feasibility assessment, budget preparation and negotiation, regulatory and IRB submission and oversight, study coordinator services and clinical trial placement.
Learn more about the Clinical Trials Office .
Biostatistics support is provided by the Division of Biostatistics and Bioinformatics in the Department of Public Health Sciences. Statisticians can assist researchers with all sizes and types of projects, from simple data analyses to large multi-center clinical trials. Specific services include grant proposal preparation, study design/sample size calculation, development of a statistical analysis plan, data analysis and interpretation, manuscript review and preparation, response to reviewer comments and statistical advice only. Learn more and access the consultation form on the CTSI website .
The Clinical Research Center (CRC) provides clinical research resources and expertise to investigators who conduct research with human subjects. The 6,800-square-foot CRC in Hershey is located on the fourth floor of Penn State Health Milton S. Hershey Medical Center and includes clinical exam rooms, private subject beds, procedure space, an observational study suite, consultation space, infusion sleep rooms, negative pressure rooms, DXA scanner and specimen processing and storage space. The unit is staffed by research nurses who implement protocol-specific requirements including drug administration, timed blood draws, electrocardiograms and assistance with various study-related procedures. They are certified in chemotherapy/biotherapy administration. CRC nursing is available 7:30 a.m. to 4 p.m. Monday-Friday. Nursing assistance may be available outside of these times with adequate notice. The unit is available by badge access to investigators and their study teams 24 hours a day.
The Exercise Research Center (ERC) is a 4,500-square-foot, state-of-the-art facility conveniently located at the Hershey Center for Applied Research (HCAR) for easy access for participants. Resources include four separate testing areas, a reception and waiting area, exam room and secure file room. The Exercise Research Center has a fee-for-service basis and provides highly skilled and trained exercise physiologists and CRC nursing support to conduct body composition and exercise assessments.
Body Composition equipment includes:
- DXA scanner
- Resting metabolic analyzer
- Bioelectrical impedance analysis (BIA)
- Anthropometric measurers
Exercise Physiology equipment includes:
- Metabolic gas analysis system
- Biodex dynamometer
- Multistation resistance training unit
- Strength training equipment
- Pulmonary function tests
- Treadmills, bikes and arm ergometers
Clinical Research Nurses: Highly skilled clinical research nurses implement protocol-specific procedures and provide direct nursing care for all subjects enrolled in research studies. CRC nurses are committed to subject safety and protocol fidelity. CRC nurses are certified in chemotherapy administration, conscious sedation and ACLS. Learn more about the CRC and request a consultation with the CRC.
The Research Ethics Consultation Service is a free service available to all biomedical researchers at Penn State who seek advice regarding ethically complex aspects of their biomedical research. Learn more on the CTSI website .
The Community Engagement Consultation Service provides opportunities for researchers and community members interested in healthcare research to get expert feedback on how to engage communities around research ideas, proposals, evaluations, and ongoing projects. Learn more on the CTSI website .
CHEER – the Community Health Equity & Engagement in Research program
CHEER is a partnership between the Social Science Research Institute (SSRI) and the Clinical and Translational Science Institute (CTSI) at Penn State. The CHEER program promotes community-engaged research (CEnR) across Penn State, spanning many disciplines, with the overall goal of enhancing wellness and reducing health disparities. It serves as the landing place for faculty who seek to engage communities in their research and for community organizations and members to engage with Penn State expertise. The CHEER team is here to jumpstart your career in CEnR, connect you with community partners based on shared interests, and provide resources and educational programming in an effort to promote meaningful and sustainable partnerships.
The CHEER Researcher Toolkit is designed to educate learners about the importance of community-engaged research (CEnR), guiding CEnR principles to support meaningful engagement and strategies to develop and maintain successful community-academic partnerships. Weaved throughout each section are real-world, evidence-based best practices and resources.
REDCap (Research Electronic Data Capture) is a secure web application for building and managing online surveys and databases. It is a novel workflow methodology and software solution designed by Vanderbilt University for rapid development and deployment of electronic data capture tools to support clinical and translational research. Using REDCap’s streamlined process for rapidly developing projects, you may create and design projects using:
- the online method from your web browser using the Online Designer
- the offline method by constructing a “data dictionary” template file in Microsoft Excel, which can be later uploaded into REDCap
Both surveys and databases (or a mixture of the two) can be built using these methods. REDCap provides audit trails for tracking data manipulation and user activity, as well as automated export procedures for seamless data downloads to Excel, PDF, and common statistical packages (SPSS, SAS, Stata, R). Also included are a built-in project calendar, a scheduling module, ad hoc reporting tools, and advanced features, such as branching logic, file uploading, and calculated fields. REDCap has a quick and easy software installation process, so that you can get REDCap running and fully functional in a matter of minutes. Learn more about REDCap on the CTSI website . The staff of the Data Management Unit at the Department of Public Health Sciences offers REDCap configuration services to allow investigators to more easily develop and implement a fully operational and customized REDCap project based on the needs of their study. Services include:
- REDCap project design ( e.g., longitudinal studies and cross-sectional surveys)
- Development of case report forms, data entry forms and surveys
- Creation of REDCap randomization models as well as backup randomization processes
- Creation of data quality rules and data flow processes
- Customization and implementation of study’s Electronic Regulatory Binder
The Trial Innovation Network is a new collaborative initiative within the Clinical and Translational Science Awards (CTSA) Program and is composed of three key organizational partners – the CTSA Program Hubs, the Trial Innovation Centers (TICs), and the Recruitment Innovation Center (RIC). All are key partners of the Trial Innovation Network and make unique and essential contributions. Other important partners include NIH institutes, other federal and non-federal stakeholders, researchers, patients, providers and the public. The local Penn State Hub Liaison Team works together to provide support and resources for investigators to develop proposals into protocols, optimize study operations, and enhance recruitment and enrollment. Investigators must contact their local Trial Innovation Liaison Team to discuss their proposal and obtain a brief consultation prior to submission. A consultation with the local Trial Innovation Liaison Team is important because these teams will directly connect the local hubs to the national network and provide advice and input on proposals. Learn more on the CTSI website and the Trial Innovation Network national site .
The Exercise Research Center provides space, equipment and trained personnel to Penn State investigators. Resources include unique facilities and equipment, as well as highly experiences staff who are trained in human subjects’ protection, good clinical practices, protocol implementation and compliance. The facilities are approximately 4,500 square feet and include a DXA scanner, resting metabolic rate system, BODPOD, anthropometric measures, skinfold calipers and bioelectrical impedance analysis. Exercise testing equipment includes stationary and portable VO2 metabolic systems, a Biodex, resting ECG, treadmills, weight machines, bikes and a multi-station, resistance training unit.
Exercise Physiologist: A highly skilled exercise physiologist staffs the ERC and can provide oversight of all of the tests that can be conducted at the facility. The ERC exercise physiologist is committed to subject safety.
Learn more about the Exercise Research Center on the Clinical Research Center website .
Study Development and Feasibility
A pool of potential study subjects can be estimated using TriNetX. Through TriNetX, users search for patients meeting specified criteria in a de-identified database, without prior Institutional Review Board (IRB) approval. Data are presented as unique patient counts, and a patient is counted only once. Data in TriNetX also exclude patients with only a medical record number or without diagnoses or codes. Such a search can help researchers determine whether enough potential patients are available to properly conduct a research study. With IRB approval and an enterprise information management request, patient-level data can be requested.
TriNetX also offers chart and graph options for data visualization and includes a rate-of-arrival algorithm. This algorithm determines how many patients matching certain criteria visited Penn State Health within the past three years, and then predicts how many potential visits will happen each quarter over the next year. A Trial Connect feature allows clinical research organizations and industry sponsors to determine and connect with potential study sites.
Learn more at Penn State Clinical and Translational Science Institute’s website .
Because it may be necessary for a researcher to obtain access to and review PHI in order to prepare a research study, HIPAA rules allow such a review upon compliance with specified criteria. In order to gain access to the PHI, the principal investigator needs to demonstrate that the use or disclosure of the protected health information is solely to prepare a research protocol or for similar purposes preparatory to research, that the researcher will not remove any protected health information from the covered entity, and representation that protected health information for which access is sought is necessary for the research purpose. Submit the Review Preparatory to Research Attestation Form for IRB for review and approval. For more information about the Privacy Rule and preparatory to research provisions go to: 45 CFR 164.512i, paragraph (1)(ii) of the definition of “protected health information.”
In order to remain compliant with the privacy laws, investigators are strongly encouraged to utilize de-identified data, when possible, and access such data through TriNetX. Investigators do not need to file this form to access de-identified data through TriNetX .
For all preparatory requests, the following rules apply:
- The investigator must attest that the work is solely to review PHI to prepare a research protocol or for similar purposes preparatory to research.
- The investigator must provide a statement affirming that no PHI will be removed from the covered entity by the researcher in the course of the review. This means that the data retrieved cannot be shared, in an identifiable fashion, with any person or third-party agency.
- The investigator may only access the information necessary to reach their research goals in accordance with the Minimum Necessary Rule.
- The investigator must agree that any access to PHI without a signed HIPAA authorization will be tracked by the individual accessing the information.
This activity is required for industry-sponsored clinical studies and investigator-initiated clinical studies with funding from industry. In many instances a sponsor sends a Confidential Disclosure Agreement (CDA) prior to sharing a protocol or confidential documents. If a CDA is not provided by industry, a PI may request that the Office of Research Affairs send a CDA to the industry representative. If a PI receives a CDA or would like to send a CDA, the request should be submitted via this online form (Penn State Access ID login required). The Office Research Affairs reviews CDAs in great detail and ensures that it complies with the Penn State Health and Penn State College of Medicine rules for confidentiality, data retention and information ownership. CDAs require an authorized signature of Penn State College of Medicine, as well as a signature from the PI acknowledging the confidentiality obligations. See the “Submitting Contracts for Approval” section of this guidebook for other industry contract-related activities.
The Department of Medicine, under the direction of Dr. Christopher Sciamanna, operates a grant preparation service for investigator-initiated clinical trials. The group works with faculty who are at the point in their career where they have some pilot data, a compelling clinical trial question to answer and the ability to do the work if funded. The team includes a PhD with experimental research training who works closely with Dr. Sciamanna and a grants management specialist to address budgetary and other grant requirements. This is not solely a grant preparation service and works closely with investigators to help them design the trial so that it has the greatest possible chance of external funding. This group focuses the grants toward large federal funders (NIH, PCORI, etc) but is not opposed to other sources, assuming they are large enough to conduct a properly powered clinical trial. The first step to using this service is to speak to Dr. Sciamanna ( [email protected] ). The group works with investigators in departments other than Medicine, and the service has been operational since 2016.
The Department of Public Health Sciences has been serving as a data coordinating center for NIH-funded multi-site clinical trials since 1993. Through this experience, the department has developed the following areas of expertise:
- Data management
- Biostatistics
- Research computing
- Project management
After consulting with you and assessing the scope of the project, an experienced individual, or a full team of qualified researchers, can be assigned to work with you to efficiently handle all aspects of your project. Services include protocol development, project management (both administrative and clinical), forms development, data management, REDCap development, custom application development, IND/IDE submissions, manual of operations (MOP) development, statistical consultations, and snalysis and manuscript preparation and review. Learn more about services from the Department of Public Health Sciences .
Some no-cost support is available through the Biostatistics, Epidemiology and Research Design (BERD) core within the Clinical and Translational Science Institute (CTSI). Request a consultation for all BERD services here .
Monitoring is the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded, and reported in accordance with the protocol, SOPs, GCPs, and the applicable regulatory requirement(s).
Typically, academic sites are familiar with monitors assigned by a sponsor or a contract research organization (CRO). However, GCP requires that investigator-initiated trials enrolling human subjects also provide a monitoring plan to assure that the data collected throughout the study are accurate. In addition, the Code of Federal Regulations requires monitoring under 21CFR 312 subpart D (for INDs) and 21CFR 812 subpart C (for IDEs).
Sponsors (including Sponsor-Investigators) of clinical investigations conducted under an IND or IDE are required to provide oversight to ensure adequate protection of the rights, welfare, and safety of human subjects and the quality and integrity of the resulting data submitted to FDA.
This oversight is maintained through the regular review of the source data, case report forms, informed consents, regulatory documents and any other essential documents by a monitor.
During a monitoring visit, a monitor reviews individual subject records and source documents, regulatory binder(s), and other essential documents and compares the information with data recorded on the case report forms (CRF) or entered in the electronic case report form (eCRF).
The monitor is obligated to ensure the following:
- Subjects meet eligibility requirements
- The rights and safety of human subjects are protected
- Informed consent has been obtained and documented appropriately
- Conduct of trial is in compliance with protocol, good clinical practice (GCP), and applicable regulatory requirements
- Subject was followed and treated according to the protocol
- Reported trial data are accurate, complete, and 100% verifiable from source documents; all pertinent information in the subject records must be accurately recorded on the CRF
- The CRF is complete, legible, and consistent throughout visits
Typically, in an industry-sponsored study, the pharmaceutical company will provide the monitor for the study. However, in the case of a study conducted by a Sponsor-Investigator, the Investigator takes on the responsibility of ensuring that the study is being monitored.
For industry-sponsored studies a monitoring plan will often be used to guide the frequency of monitoring visits to investigative sites whereas in an Investigator-initiated study the Investigator and/or study staff should develop a monitoring plan.
The frequency of visits is affected by the complexity of the study and the rate of enrollment. Monitoring plans can be updated during the course of the study if, for example, enrollment is faster than expected. When a monitor comes to a clinical site to conduct a monitoring visit, they will need access to all source documents, including the Electronic Medical Record (EMR).
Learn more about the process for requesting monitoring access to the EMR via the Clinical Trials Office .
Clinical Trial Monitoring Services are required by Penn State College of Medicine Institutional Review Board (IRB) and Research Quality Assurance (RQA) offices for investigational drug/devices (IND/IDE), multisite and high-risk clinical trials. The Data Management Unit of the Department of Public Health Sciences receives institutional support to provide no-cost monitoring services, as directed by the IRB and RQA offices, to Penn State investigators conducting clinical research. Additionally, budget estimates can be prepared for other types of human subject research interested in data monitoring services. Estimates should be obtained early in the protocol development and feasibility process. Clinical Trial Monitoring services provided by the Data Management Unit help the investigator ensure that:
- All clinical trial activities follow the research protocol
- Participants’ rights, welfare, and safety are protected
- Quality, reliability, and integrity of data collected are maintained throughout the study
- Liability risk to the institution is minimized
Services include:
- Creation of a customized data monitoring plan
- Source document verification
- Review of regulatory documents
- Tracking of investigational products
- Data monitoring visits
See the Department of Public Health Sciences for details .
This section describes the roles, responsibilities and operating procedures of Data Monitoring Committees (DMCs) (also known as Data and Safety Monitoring Boards (DSMBs) or Data and Safety Monitoring Committees (DSMCs) that may carry out important aspects of clinical trial monitoring. A clinical trial Data Monitoring Committee is a group of individuals with pertinent expertise that reviews on a regular basis accumulating data from one or more ongoing clinical trials. The DMC advises the investigator regarding the continuing safety of trial subjects and those yet to be recruited to the trial, as well as the continuing validity and scientific merit of the trial. DMCs have the practical position of seeing data and safety information in more frequent intervals and with typically more statistical expertise to make enhanced assessments about a study’s progress and determine the study’s future.
DMC/B: What do they do?
DMC/Bs perform the following general functions:
- Objectively appraise a study’s progress
- Assess data quality via a formal and planned process
- Provide analytical expertise and rigor
- Determine the statistical significance of efficacy and/or risk‐benefit ratio
- Serve as “another set of eyes”
In accordance with its analytic and ethical responsibilities, a DMC is tasked to determine whether a study can proceed with enrollment, as designed. It has the authority to halt a study, suspending enrollment, pending crucial changes to the protocol’s design, recruitment strategy, risk minimization, or other modification. It can also terminate a study due to statistically significant efficacy or increased risk of harm to participants.
DMC/Bs: When are they needed?
A fundamental reason to establish a DMC/B is to enhance the safety of trial participants in situations, in which safety concerns may be unusually high, in order that regular interim analyses of the accumulating data are performed. All clinical trials require safety monitoring, but not all trials require monitoring by a formal DSMC/B. DMC/Bs are established for large, randomized multisite studies that evaluate treatments intended to prolong life or reduce risk of a major adverse health outcome such as a cardiovascular event or recurrence of cancer. DMC/Bs are generally recommended for any controlled trial of any size that will compare rates of mortality or major morbidity. Formal data and safety monitoring is also necessary to assure confidence in a study’s interim and final outcomes:
- To verify or validate efficacy and/or safety information significant to a novel therapy
- To gauge data quality to confirm the research question/ hypothesis in developing treatments
- To assess efficacy and safety when “lives and wellbeing depend on valid results”
The FDA recommends that sponsors consider using a DMC/B when:
- The study endpoint is such that a highly favorable or unfavorable result, or even a finding of futility, at an interim analysis might ethically require termination of the study before its planned completion
- There are a priori reasons for a particular safety concern, as, for example, if the procedure for administering the treatment is particularly invasive
- There is prior information suggesting the possibility of serious toxicity with the study treatment
- The study is being performed in a potentially fragile population such as children, pregnant women or the very elderly, or other vulnerable populations, such as those who are terminally ill or of diminished mental capacity
- The study is being performed in a population at elevated risk of death or other serious outcomes, even when the study objective addresses a lesser endpoint
- The study is large, of long duration, and multi-center
In studies with one or more of these characteristics, the additional oversight provided by a DMC/B can further protect study participants. In other studies, such as short-term studies for relief of symptoms as noted above, such committees are generally not warranted. ( FDA Guidance: The Establishment and Operation of Clinical Trial Data Monitoring Committees for Clinical Trial Sponsors – Guidance for Clinical Trial Sponsors – Establishment and Operation of Clinical Trial Data Monitoring Committees )
DMC/B Charters
DMC/Bs typically operate under a written charter that includes well-defined standard operating procedures. Such charters are important for the same reason that study protocols and analytical plans are important—they document that procedures were pre-specified and thereby reduce concerns that operations inappropriately influenced by interim data could bias the trial results and interpretation. The sponsor may draft this charter and present it to the DMC/B for agreement, or the DMC/B may draft the charter with subsequent concurrence by the sponsor. Topics to be addressed would normally include a schedule and format for meetings, format for presentation of data, specification of who will have access to interim data and who may attend all or part of DMC/B meetings, procedures for assessing conflict of interest of potential DMC/B members, the method and timing of providing interim reports to the DMC/B, and other issues relevant to committee operations. FDA may request that the sponsor submit the charter to FDA well in advance of the performance of any interim analyses, ideally before the initiation of the trial (see 21 CFR 312.23(a)(6)(iii)(g); 21 CFR 312.41(a); 21 CFR 812.150(b)(10)). In such cases, FDA would usually consider the charter when FDA reviews the study protocol. ( FDA Guidance: The Establishment and Operation of Clinical Trial Data Monitoring Committees for Clinical Trial Sponsors – Guidance for Clinical Trial Sponsors – Establishment and Operation of Clinical Trial Data Monitoring Committees )
The Department of Radiology supports and encourages clinical research at Penn State Health and Penn State College of Medicine. If your protocol contains ionizing radiation procedures (standard of care and/or research-related procedures), you must complete the Radiation Review Form and include it with your IRB submission in CATS IRB. All research protocols/studies that involve non-routine radiation procedures must be reviewed by the Human Use of Isotope Committee (HUIC). It is also highly advised that you communicate with the CTO prior to starting your study or even at the protocol preparation step. CTO will work with Radiology to establish the exact process for your procedure and will provide a cost estimate. This is especially important if your experimental requirements deviate from the standard radiology procedures, i.e. require an unusual contrast agent. It is not uncommon that radiology services are not clearly detailed in the text of the protocol, potentially resulting in additional unanticipated charges at the point of service. See the Additional Approvals in the “Preparing Documents” section of this guidebook for details. HRP 903 Radiation Review Form is available in the CATS IRB library (login required).
The Department of Pathology is committed to excellence in patient care, education, and translational and basic research, and plays a vital and integral role in the medical center’s multifaceted missions. Through provision of high quality diagnostic services and the practice of laboratory medicine, the department supports the wide range of medical care provided at the institution.
Find Laboratory Licenses (such as CLIA) and Accreditations via the Clinical Trials Office .
Investigators planning to use any of the following specimens in their research project must complete the Use of Human Tissue for Research Form and include it with the CATS IRB Submission:
- Collection of tissue from surgical or biopsy procedures
- Collection of bone marrow from bone marrow biopsies and/or bone marrow aspirates
- Use of archival pathologic specimens stored in Anatomic Pathology
- Collection of tissue from cadavers
- Collection of placenta specimens
The completed form is reviewed by Anatomic Pathology during IRB review. After review and approval of the proposed tissue request, the Department of Pathology will notify the investigator and the IRB. Pathology approval will be required before the official IRB approval is issued.
No tissue will be released to an investigator without approval from Anatomic Pathology. See the Additional Approvals item in the “Preparing Documents” section of this guidebook for details.
General Information on Pathology
Any time human tissue is removed for research purposes, the specimen must pass through either the Surgical Pathology Laboratory or Autopsy Suite. Adequate diagnostic tissue will be retained in the laboratory prior to providing specimens for research. Therefore, Pathology is usually able to provide tissues from major surgical excisions but can only rarely provide tissues from small biopsies obtained for diagnostic purposes.
There is a charge per specimen to help defray the technical cost in obtaining research tissues. Please call the Department of Pathology at 717-531-8352 for the current charge to use when preparing the budget for a grant.
If the investigator is aware that a biopsy or excision of the desired tissue is scheduled, they should complete and submit to the Anatomic Pathology Gross Room (fax to 717-531-0831) a “Research Tissue Request Form” prior to the scheduled date of surgery. This form notifies the Gross Room staff that a specimen is a potential source of research tissue so that the specimen can be handled appropriately for that purpose.
A blank copy of this form can be obtained from the Gross Room; this blank form can be photocopied for multiple submissions.
See the “Investigational Drug Pharmacy (IDS)” section in this guidebook for a detailed description of the Investigational Drug Services (IDS), start-up requirements and fees.
The mission of the Clinical Research Biospecimen Core is to provide a centralized service for the processing and distribution of human subject research samples for all clinical research activities at Penn State College of Medicine and Penn State Health Milton S. Hershey Medical Center.
The goals of the Clinical Research Biospecimen Core (CRBC) are two-fold:
- First, by providing a centralized service for clinical research sample handling, more favorable study contract pricing with extramural research sponsors will be allowed.
- Second, by utilizing a centralized core service dedicated solely to this purpose, internal investigators will find it easier to budget and efficiently conduct their human subject research efforts.
IND and IDE Submissions
FDA’s Center for Drug Evaluation and Research (CDER) is responsible for regulating manufacturing, testing and importation of pharmaceutical drugs in the US. This includes new drug approvals, abbreviated new drug approvals (generics), over-the-counter drugs, animal drugs and biologics. A drug is defined as:
- substance intended for use in diagnosis, cure, mitigation, treatment, or prevention of the disease;
- substances (other than food)intended to affect the structure or any function of the body;
- substance intended for use as a component of a medicine but not a device or component of a device.
Other sections of this guidebook provide a brief summary of regulatory requirements for clinical research involving drugs, biologics or dietary supplements.
Preclinical testing begins after a potential drug has been identified in the lab. Preclinical testing involves lab and animal studies that evaluate the drug’s toxic and pharmacologic effects. Preclinical studies are also subject to the FDA regulations known as Good Laboratory Practices (GLP) for Nonclinical Laboratory Studies, 21 CFR 58. The GLP regulations specify minimum standards in such areas as personnel, facilities, equipment and operations.
Pre-clinical studies not performed under GLP conditions may not be accepted by the FDA. Recognition of this fact is particularly important for academic drug development. Please see “Roadmap for Academic Health Centers to Establish Good Laboratory Practice-Compliant Infrastructure”, Acad. Medicine, 2012 87(3):279-284
Preclinical testing includes pharmacokinetics, the study of how the drug moves through living organisms. Researchers examine absorption, distribution, metabolism and excretion (also abbreviated as ADME) to ensure that the drug reaches its intended target and passes through the body properly. In addition to the biological tests, researchers conduct chemistry tests to establish the drug’s purity, stability and shelf life. Manufacturing tests are conducted to determine the feasibility of producing the drug on a large scale and to explore dosing, packaging and formulation (e.g., pill, inhaler, injection). At the preclinical stage, the FDA will generally ask, at a minimum, that sponsors:
- develop a pharmacological profile of the drug;
- determine the acute toxicity of the drug in at least two species of animals, and
- conduct short-term toxicity studies ranging from two weeks to three months, depending on the proposed duration of use of the substance in the proposed clinical studies.
FDA Guidance for Industry: See Content and Format of Investigational New Drug Applications (INDs) for Phase 1 Studies of Drugs, Including Well-Characterized, Therapeutic, Biotechnology-derived Products .
After preclinical testing is completed, a sponsor or sponsor-investigator (see below) files an IND with FDA prior to beginning any human testing. An IND is a request for FDA authorization to administer an investigational drug or biological product to humans. Such authorization must be secured prior to interstate shipment and administration of any unapproved drug or biological product that is not the subject of an approved New Drug Application or Biologics/Product License Application. The application must show results of preclinical experiments; the chemical structure of the compound; how it is thought to work in the body; any side effects found in animal studies; and how the compound is manufactured (chemistry, manufacturing and controls section). The IND must also include a detailed clinical trial plan, including how, where and by whom the studies will be conducted. Based on the information of the IND application, the FDA will determine if there is sufficient evidence to support initial human testing. The sponsor must wait 30 days after submitting the IND to the FDA for review. At the end of the 30 day review period, unless the FDA identifies a potential safety problem involving the use of the drug and asks for a delay, the sponsor may begin the proposed clinical testing. Per Penn State University Policy RP-05 “Research Quality in Human Participant Research,” it is required that the Research Quality Assurance (RQA) group be contacted to provide support for the submission process for INDs or IND Exemptions, and to perform an administrative review of the submission prior to being sent to the FDA. See Penn State University policy .
The terms “expanded access,” “compassionate use,” “treatment use” and “treatment IND” are used interchangeably to refer to use of an investigational drug when the primary purpose is to diagnose, monitor, or treat a patient’s disease or condition. Investigational drugs are new drugs that have not yet been approved by the FDA or approved drugs that have not yet been approved for a new use, and are in the process of being tested for safety and effectiveness. The distinction between administering an investigational drug in the setting of a “traditional” clinical trial versus expanded access lies in the intention. In a traditional clinical trial the intention is to understand the safety and effectiveness of the investigational drug; in expanded access the intention is treatment. There are four general guidelines for a drug to be considered for expanded-access use:
- Patients with a serious or immediately life-threatening disease or condition, where there is no comparable or satisfactory alternative therapy to diagnose, monitor, or treat the disease or condition.
- No comparable or satisfactory alternative therapy exists.
- The potential patient benefit justifies the potential risks of the treatment, and those risks are not unreasonable in the context of the disease or condition being treated.
- The expanded use of the investigational drug for the requested treatment will not interfere with the initiation, conduct, or completion of clinical investigations that could support marketing approval of the product.
The Human Research Protection Program (HRPP) should be contacted for further guidance as soon as a provider is considering using an investigational drug under any expanded access condition. See FDA details on expanded access .
After clinical trials have been completed demonstrating safety and effectiveness, the study sponsor (or drug manufacturer) will submit a New Drug Application (NDA) to the FDA for a license to market the drug for a specified indication. NDAs contain all of the information about all of the studies, including preclinical testing, all clinical trials, dosing information, manufacturing details and proposed labeling for the new medicine. Most academic drug development efforts do not progress to this stage. At NDA submission stage, FDA scientists review all the results from all the studies carried out over the years and determine if they show that the medicine is safe and effective enough to be approved. During this review, the FDA determines what the labeling should be and whether the sponsor can manufacture it properly and consistently. Once the drug is approved, it becomes available for physicians to prescribe for the indication approved by the FDA. The review process takes approximately 18 months.
Many academic investigators will want to conduct a clinical study with an already approved drug. This is often done to establish efficacy in a new disease indication. FDA provides provisions for conducting studies of lawfully marketed drugs through the use of an IND exemption. A clinical investigation of a drug is exempt from the IND requirements if all of the criteria for an exemption in 21CFR312.2(b) are met:
- The drug product is lawfully marketed in the United States;
- The investigation is not intended to be reported to FDA as a well-controlled study in support of a new indication and there is no intent to use it to support any other significant change in the labeling of the drug;
- The investigation is not intended to support a significant change in advertising to an existing lawfully marketed prescription drug product;
- The investigation does not involve a route of administration or dosage level or use in a patient population or other factor that significantly increases the risks (or decreases the acceptability of the risks) associated with the use of the drug product;
- The investigation will be conducted in compliance with the requirements for institutional review set forth in FDA regulations 21 CFR 56, and requirements for informed consent as set forth in FDA regulations 21 CFR 50;
- The investigation will be conducted in compliance with FDA regulations 21 CFR 312.7: Promotion of investigational drugs.
Thorough documentation is required to support this exemption criterion and may include prior publications or other public disclosures. If such evidence cannot be provided, a physician should submit a research IND (limited in scope) to the FDA. The physician is now considered sponsor-investigator. FDA Guidance: See Investigational New Drug Applications (INDs) – Determining whether Human Research Studies can be conducted without an IND . Per Penn State University Policy RP-05 “Research Quality in Human Participant Research,” it is required that the Research Quality Assurance (RQA) group be contacted to provide support for the submission process for INDs or IND Exemptions, and to perform an administrative review of the submission prior to being sent to the FDA. See Penn State University policy .
Many clinical studies of academic investigators evaluate the effect of dietary supplements on the disease or physiological parameters. Some of these studies may require an IND submission. If the dietary supplements are investigated for diagnosis, cure, mitigation, treatment, or prevention of disease and are used to affect the structure or function of the body, then the dietary supplement will be considered a drug for the purposes of this study. The study will be the subject to the same regulations as any other unapproved drug. Specifically, the FDA will be paying particular attention to the composition of the dietary supplement, its origin and manufacturing processes. When preparing the INDs for dietary supplements, make sure that the supplement manufacturer is willing to provide this information. FDA Guidance: See Investigational New Drug Applications (INDs) – Determining whether Human Research Studies can be conducted without an IND .
FDA’s Center for Devices and Radiological Health (CDRH) is responsible for regulating manufacturing and importation of medical devices sold in the United States. In addition, CDRH regulates radiation-emitting electronic products (medical and non-medical) such as lasers, X-ray systems, ultrasound equipment, microwave ovens and color televisions.
If a product is labeled, promoted or used in a manner that meets the definition in section 201(h) of the Federal Food Drug & Cosmetic (FD&C) Act, it will be regulated as a medical device.
A device is: “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is:
- “intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals,” or
- “intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.”
This definition provides a clear distinction between a medical device and other FDA regulated products such as drugs. If the primary intended use of the product is achieved through chemical action or by being metabolized by the body, the product is usually a drug. In cases where it is not clear whether a product is a medical device the Division of Small Manufacturers, International and Consumer Assistance (DSMICA) can assist in making a determination.
FDA Guidance: See Frequently Asked Questions about Medical Devices .
See details via the Clinical Trials Office .
The FDA has established classifications for approximately 1,700 different generic types of devices and grouped them into 16 medical specialties referred to as panels. Each of these generic types of devices is assigned to one of three regulatory classes (Class I, Class II and Class III) based on the level of control necessary to assure the safety and effectiveness of the device.
The device classification defines the regulatory requirements for an approval of a new device. Regulatory control increases from Class I to Class II to Class III. Device classification depends on the intended use of the device and also upon indications for use. In addition, classification is risk-based, that is, the risk the device poses to the patient and/or the user is a major factor in the class it is assigned.
- Class I devices: elastic bandages, examination gloves and hand-held surgical instruments.
- Class II devices: powered wheelchairs, infusion pumps and surgical drapes.
- Class III devices: implantable pacemaker pulse generators and coronary stents.
To find the classification of a device, as well as whether any exemptions may exist, the classification regulation number should be determined for the device. One of the ways to accomplish this is to go directly to the FDA classification database and search for a part of the device name. Once the correct classification regulation has been identified, go to the device panel (medical specialty) to which the device belongs .
The search will provide the Device Classification and the appropriate CFR regulation for the device. If the device is not classified, similar devices can be researched on the FDA website (PMA and 510(k) databases) or pre-IDE consultation can be used for the FDA determination. The CTO has additional device information specific to our Medicare Administrative Contractor (MAC), Novitas Solutions, Inc.
Devices used on human subjects to conduct investigations of safety and effectiveness are considered “Investigational Devices” (Section 520(g) of FD&C Act). Significant Risk (SR) device presents a potential for serious risk to the health, safety and welfare of a subject, and:
- Intended to be used as an implant
- Purported to support or sustain human life
- Is used for substantial importance in diagnosing, curing, mitigating or treating disease, or
- Otherwise presents a potential for serious risk to the health, safety, or welfare of a subject.
Examples of SR devices include sutures, cardiac pacemakers, hydrocephalus shunts, and orthopedic implants. Conversely, non-significant risk (NSR) device studies do not pose a significant risk to patients. Non-significant risk should not be confused with “minimal risk,” a term used by the FDA to classify studies. Examples of NSR devices include most day-wear contact lenses and lens solutions, ultrasonic dental scalers, and foley catheters. SR devices must meet all regulatory requirements set in 21 CFR 812, including the requirement for approval by both IRB and the FDA before commencing the study. Significant risk devices require submission of an investigational device exemption (IDE) to CDRH. NSR device studies may commence without FDA approval, based solely on the IRB approval. However, the sponsor-investigator must follow abbreviated IDE requirements, which are, in essence, the same requirements as regular IDE only without FDA submission (21 CFR 812.2 (b)). The IRB acts as a surrogate overseer for the FDA. FDA Guidance: See Significant Risk and Non-significant Risk Medical Device Studies .
Important: Clinical study of a new indication or new patient population for an already marketed device falls under the IDE regulations. Per Penn State University Policy RP-05, “Research Quality in Human Participant Research,” it is required that the Research Quality Assurance (RQA) group be contacted to provide support for the submission process for IDEs or Study Risk Determinations, and to perform an administrative review of the submission prior to being sent to the FDA. See Penn State University policy . An investigational device exemption (IDE) is a regulatory submission to the CDRH. If approved, it allows the investigational device to be used in a clinical study in order to collect safety and effectiveness data. IDE requirements:
- Study approved by an institutional review board (IRB). If the study involves a significant risk device, the IDE must also be approved by FDA;
- Informed consent from all patients obtained and documented;
- The device is labeled “CAUTION- Investigational Device. Limited to investigational use only;”
- Sponsor-investigator complies with monitoring requirements;
- Records and reports are maintained;
- Investigator cannot promote or commercialize (charge for) the device.
FDA Guidance: See Investigational Device Exemptions (IDEs) for Early Feasibility Medical Device Clinical Studies, Including Certain First in Human (FIH) Studies . Two important elements of the guidance are:
- FDA approval of an IDE application for an early feasibility study, including some first-in-human studies, may be based on less nonclinical data than would be expected for other types of studies (e.g., traditional feasibility or pivotal).
- more types of modifications that can be made under a 5-day notification without prior FDA approval, as compared with other types of studies;
- a contingent approval process that permits changes contingent upon acceptable
- nonclinical test results without requiring additional FDA action; and
- interactive review of IDE supplements and amendments.
Studies of non-significant risk devices are subject to abbreviated IDE requirements. An IDE submission to the FDA is not required under the abbreviated requirements, but the requirements for labeling, IRB approval, informed consent, monitoring, records, reports and promotional practices contained in FDA regulations still apply (21 CFR 812.2(b)). In addition, the concept of “non-significant risk” to determine whether abbreviated IDE procedures are appropriate should not be confused with “minimal risk” to determine whether expedited IRB review is appropriate. For a device study to be eligible for expedited IRB review, it must be a non-significant risk device AND present no more than minimal risk to the subject (ref. 21 CFR 56.110). Requirements under abbreviated IDE:
- The sponsor will label the device in accordance with 21 CFR 812.5.
- The sponsor will obtain and maintain IRB approval throughout the investigation as a nonsignificant risk device.
- The sponsor will ensure that each investigator participating in an investigation of the device obtains and documents consent from each subject under the investigator’s care according to 21 CFR 50, unless documentation is waived by an IRB.
- The sponsor will comply with the requirements of 21 CFR 812.46 with respect to monitoring investigations.
- The sponsor will maintain the records required under 21 CFR 812.140(b) (4) and (5) and make the reports required under 21 CFR §812.150(b) (1) – (3) and (5) – (10); The sponsor will ensure that participating investigators will maintain the records required by 21 CFR 812.140(a)(3)(i) and make the reports required under 812.150(a) (1), (2), (5), and (7).
- The sponsor will comply with the prohibitions in 21 CFR 812.7 against promotion and other practices.
Some studies may be exempt from the IDE regulations. The exemption criteria is explained in 21 CFR 812.2(c), and briefly summarized here:
- A legally marketed device when used in accordance with its labeling;
- noninvasive;
- does not require an invasive sampling procedure that presents significant risk;
- does not by design or intention introduce energy into a subject;
- and is not used as a diagnostic procedure without confirmation of the diagnosis by another medically established diagnostic product or procedure.
- Consumer preference testing, testing of a modification, or testing of a combination of two or more devices in commercial distribution, if the testing is not for the purpose of determining safety or effectiveness and does not put subjects at risk;
- A device intended solely for veterinary use;
- A device shipped solely for research with laboratory animals;
- A custom device as defined in 812.3(b).
An unapproved medical device is a device that is utilized for a purpose, condition, or use for which the device requires, but does not have, an approved application for premarket approval or an approved IDE. Emergency use is permitted if the treating physician determines that:
- The patient has life-threatening condition that needs immediate treatment
- No generally acceptable alternative treatments exist
- Because of an immediate need to use the device, there is no time to use existing procedures to get FDA approval
Next, the treating physician needs to undertake the following protective measures:
- An independent assessment by an uninvolved physician
- Informed consent from the patient or legal representative
- Clearance from the institution as specified by their policies
- Approval of the IRB Chair
- Approval from the IDE sponsor, if an approved IDE exists for the device
- Prior FDA approval for shipment or emergency use of the investigational device is not required, but the use should be reported to the FDA by the IDE sponsor via a supplement within 5 working days from the time the sponsor learns of the use.
Note that if a physician who is faced with an emergency situation contacts the FDA to discuss their patient’s condition, in this situation the FDA will only act in an advisory role, rather than in an approving role. The responsibility for making the decision as to whether the situation meets the emergency use criteria and whether the unapproved device should be used lies with the physician. FDA Guidance: See Emergency Use Authorization of Medical Products . See detailed guidance about what to submit to the IRB/HSPO .
This type of use is not an emergency use. The compassionate use (or Single Patient/Small Group Access) provision allows access for patients who do not meet the requirements for inclusion in a clinical investigation but for whom the device may provide a benefit in treating and/or diagnosing their disease or condition. This provision is typically approved for individual patients but may be approved to treat a small group. Compassionate use requires the submission by the sponsor or investigator of an IDE Supplement as per 21 CFR 812.35 requesting approval in order to treat the patient(s). In order to permit this use, FDA will review the following information:
- The patient’s condition and the circumstances necessitating treatment
- Whether comparable alternative treatment exists, and the probable risk of using the investigational device is no greater than the probable risk from the disease or condition
- The protocol to be followed, or deviations from the approved clinical protocol that may be needed in order to treat the patient
- The patient protection measures that will be followed (informed consent, concurrence of IRB chairperson, clearance from the institution, independent assessment from uninvolved physician, authorization from IDE sponsor)
- Whether the preliminary evidence of safety and effectiveness justifies such use
- Whether such use would interfere with the conduct of ongoing clinical investigations
The investigator should not treat the patient identified in the supplement until FDA approves use of the device under the proposed circumstances.
This type of use is not an emergency use. A request for Treatment Use of an investigational device in the mitigation, diagnosis and treatment of a serious disease requires a Treatment IDE Submission as per 21 CFR 812.36. If approved, Treatment IDE enables a wider group of patients to receive the investigational device for the same indication as it is being studied under the sponsor IDE. During the course of the clinical trial, if the data suggests that the device is effective, then the trial may be expanded to include additional patients with life-threatening or serious diseases. Treatment IDE will remain open even after the sponsor trial has been completed. The following provisions have to be met:
- Life-threatening or serious disease or condition
- Device is investigated in a controlled clinical trial under IDE for the same use
- Sponsor is actively pursuing market approval
- No comparable alternative treatment exists
- The device is under investigation in a controlled clinical trial for the same use under an approved IDE, or such clinical trials have been completed.
Premarket approval (PMA) (21 CFR 814.39) is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. Due to the level of risk associated with Class III devices, FDA requires sufficient valid scientific evidence to assure that the device is safe and effective for its intended use(s). The content of PMA is similar to the NDA for new drugs, and contains manufacturing sections, pre-clinical laboratory studies and clinical investigations. Some devices (from Class I or Class II) may be able to be cleared under a different pathway referred to as premarket notification, or 510(k). The name refers to requirements outlined in section 510(k) of Food, Drug and Cosmetics Act. If the device is considered to be substantially equivalent to one or more similarly marketed devices (known as “predicate” devices), a claim of substantial equivalence can be made. A claim of substantial equivalence does not mean the new and predicate devices must be identical. Substantial equivalence is established with respect to intended use, design and other parameters.
A Humanitarian Use Device (HUD) is a “medical device intended to benefit patients in the treatment or diagnosis of diseases or conditions that affect or are manifested in fewer than 4,000 individuals in the United States per year.” (21 CFR 814). The request for HUD designation is described in the following FDA guidance: Humanitarian Use Device (HUD) Designations . The first step in seeking marketing approval of a HUD involves obtaining HUD designation of the device from FDA’s Office of Orphan Products Development. If the HUD request is granted, the investigator proceeds with the second step by submitting of a Humanitarian Device Exemption (HDE) application to the Office of Device Evaluation (ODE), Center for Devices and Radiological Health (CDRH), the Center for Biologics Evaluation and Research (CBER), or the Center for Drug Evaluation and Research (CDER), as applicable. An HDE is similar in both form and content to a premarket approval (PMA) application, but is exempt from the effectiveness requirements of a PMA. An HDE application is not required to contain the results of scientifically valid clinical investigations demonstrating that the device is effective for its intended purpose. The application, however, must contain sufficient information for FDA to determine that the probable benefit to health outweighs the risk of injury or illness, taking into account the probable risks and benefits of currently available devices or alternative forms of treatment. Additionally, the applicant must demonstrate that no comparable devices are available to treat or diagnose the disease or condition, and that they could not otherwise bring the device to market.
Sponsor-Investigator responsibilities under an IND or IDE are covered in 21 CFR Part 312 (for drugs) and 21 CFR Part 812 (for devices). FDA Guidance: Search for Investigator Responsibilities – Protecting the Rights, Safety and Welfare of Study Subjects .
The Research Quality Assurance (RQA) Group is available to assist with IND/IDE preparation, submission and maintenance.
Learn more about RQA .
The process and format for submitting an IND application is defined in 21 CFR 312.23, “IND Content and Format.”
The process and format for submitting an IDE application is defined in 21 CFR 812.20, “Investigational Device Exemptions – Application.”
Per Penn State University Policy RP-05, “Research Quality in Human Participant Research,” it is required that the Research Quality Assurance (RQA) group be contacted to provide support for the submission process for IDEs or Study Risk Determinations, and to perform an administrative review of the submission prior to being sent to the FDA.
See Penn State University policy .
See RQA contact details .
Preparing Documents
The purpose of these SOPs is to provide guidance to research personnel on how a clinical trial payer Coverage Analysis (CA) and budget negotiation process is under-taken in order to receive institutional and departmental approvals.
All clinical trials involving human subjects with potentially billable items and services, regardless of the funding source, should have a CA performed. Simply stated, a CA is required for studies that include services billable to insurance (i.e., when it is possible for a charge to be captured in the billing system).
The CA is not needed if a trial uses existing specimens or involves collecting data based on clinical progression. A survey, retrospective or observational study only includes a collection of forms during the patient’s standard of care. Since form collection is not billable to insurance, a CA is not required. The CA is necessary to assist in determining the responsibility of charges in a clinical trial.
A CA is a systematic review of study-related documents to determine the Medicare billing status of both the study and the items/services provided to research participants as part of a clinical research study. The CA process involves the following steps:
- Identifying studies required to undergo CA.
- Creating of the Coverage Analysis Review memo.
- Performing the “qualifying status” of the clinical trial.
- Identifying Routine Costs.
- Constructing the Study Billing Grid.
- Providing the Study Billing Grid as a tool for study team use.
Primary objective: To ensure all costs of a clinical trial are billed to the appropriate payer (sponsor, alternate funding source, institution/department, third-party payer, or participant) Medicare rules are used for various reasons. Foremost, it is not practical to budget on non-Medicare rules since Medicare drives the reimbursement rules in the United States. Medicare incorporates the “most favored nation” clause. This means that if a Medicare patient is enrolled in a clinical research study, the best deal must be given to the Medicare participant. Medicare rules for research coverage are being adopted by commercial payers, with many states already requiring commercial payers to follow rules similar to Medicare. Even pediatric studies go through the CA process since budget negotiations are based off of the coverage analysis results.
There are multiple benefits to performing a CA. It affords the institution an approach to tease out research-only charges from those items/services that are routine and/or customary care. Used as an asset in preparing a budget, it provides opportunities for increased revenue. Early detection of items/services that are not covered allows for appropriate negotiation. Additionally, we need to know the billing status of all trial procedures in order to perform a proper informed consent process. Participants are entitled to know what their financial responsibility will be during a clinical trial. Finally, it is necessary for compliant research billing processes. Errors in billing Medicare for items/services relative to clinical trials could result in allegations under the False Claims Act. Substantial fines and penalties may result. Loss of trust by sponsors and participants as well as loss of government funding may occur. Since clinical research often takes place in conjunction with the routine clinical care of patients, it is imperative to ensure that billing for both routine and research services/items are handled appropriately and in compliance with all applicable statutory requirements.
Determining coverage involves a multi-phased approach.
- If it is possible for a charge to be captured in the billing system, then a CA is performed.
- Examples of studies not requiring a CA: existing specimens, data collection on clinical progression.
- Initiate a Coverage Analysis Review memo to document and memorialize the evolution of coverage decisions.
Part 1: Identify if a clinical trial “qualifies” for Medicare coverage
- Non-device trials: Consult The Centers for Medicare and Medicaid Services (CMS) for coverage requirements (NCD 310.1: National Coverage Determination for Routine Costs in Clinical Trials).
- Device trials: CMS has established regulations for coverage of device trials. Consult the Code of Federal Regulations 42CFR 405.201 – 405.215 and 411.215 and 411.406. Additional information can be found on the Medicare Administrative Contractor’s website for our jurisdiction .
Part 2: Pinpoint items/services that are “routine costs” in the study and potentially billable
- Items or services that are typically provided absent a clinical trial (e.g., conventional care);
- Items or services required solely for the provision of the investigational item or service (e.g., administration of a non-covered chemotherapeutic agent, or surgery to implant an investigational device);
- Items or services required for the clinically appropriate monitoring of the effects of the item or service, or the prevention of complications (e.g., additional labs to monitor for side effects of the investigational product); and,
- Items or services needed for reasonable and necessary care arising from the provision of an investigational item or service, in particular for the diagnosis or treatment of complications.
Part 3: Review all statutes, regulations, national and local coverage determinations, Medicare manuals, and specialty specific practice guidelines
Analyzing items/services is solely done for billing purposes, not to judge what the provider should/should not be doing.
Further Details
Medicare/insurance will not cover items and services that are paid for by the sponsor, promised free in the informed consent document, not ordinarily covered by Medicare, and items/services used solely to satisfy data collection or analysis needs. In certain very specific instances CMS can be billed when the study is non-qualifying such as those trials that involve NO changes to medical management or treatment (i.e., observational, registry, or head-to-head). These types of quality trials do not fall under the scope of NCD 310.1. Every effort must take place to ensure that the usual, routine, medically necessary item is not provided based on protocol requirements, but rather would have been provided by the provider even in the absence of study participation.
Modifiers: A claim that contains an “investigational clinical service” must use the Q0 modifier on the HCFA 1450 form (for facilities) or on the HCFA 1500 form (for physicians). A claim that contains a “routine clinical service” must use the Q1 modifier on the forms. When Q1 is billed in conjunction with the ICD-10, Z00.6 diagnosis code, the Q1 modifier will serve as the provider’s attestation that the service meets the Medicare coverage criteria; i.e. was furnished to a beneficiary who is participating in a Medicare qualifying clinical trial and represents routine patient care, including complications associated with qualifying trial participation. National Clinical Trial (NCT) identifier or Clinicaltrials.gov Number: CMS requires ClinicalTrials.gov number on the claim when billing “routine costs” during a “qualifying clinical trial.” For clinical trial/registry/study claims with dates of service on and after January 1, 2014, this 8-digit clinical trial number must be included or claims will be returned as unprocessable. Study teams are responsible for providing sufficient information to add these identifiers to the claims. Medicare Advantage Plans (MAPs): If your study enrolls patients on the Medicare Advantage Plan, you need to be aware of special requirements for copays and claims processing. See the Medicare Managed Care Manual for details .
- Non-Device Trials: When enrolled in a qualifying clinical trial, Medicare pays for covered services as if in the original, traditional Medicare program (fee-for-service). Providers should split outpatient MAP claims and route the protocol-related “routine care” to traditional Medicare. Additionally, MAPs are responsible for copayments related to services paid under the traditional Medicare rules.
- The MAP is responsible for payment of routine care items and services in CMS-approved Category A IDE studies.
- The MAP is responsible for payment of routine care items and services, and potentially the Category B device under study in CMS-approved Category B IDE studies.
Education materials are available via the College of Medicine Clinical Trials Office’s section of this website. Additionally, in-person Clinical Research Skills Workshops are held annually.
While the clinical sites typically provide medical treatment to the subjects sustaining injury/complication on the study, who will cover the costs may not always be a clear decision. Industry sponsor, insurance or even self-pay options are considered. For privately sponsored studies that are conducted pursuant to a private sponsor’s protocol (industry sponsor), the sponsor of the study is required to pay for the reasonable cost of treating injuries or complications resulting from participation in the study, including injuries or complications resulting from the study material or research procedures performed pursuant to the study protocol, to the extent that injuries/complications were not a result of negligence, willful misconduct or failure to reasonably act on the part of the study personnel. Other costs that are incurred during conduct of the study but not resulting from the subject’s participation (i.e., typical for this type of disease or procedure) may be billed to private and government insurers, if consistent with their policies. However, in the case of injuries resulting from the natural progression of a disease or illness, the sponsor would be responsible for any injuries if, and to the extent, the progression resulted from participation in the study. In some cases, determination of whether the complication was directly or indirectly related may not be clear.
If an investigational medication is administered via an intravenous infusion, and the needle entry site became infected, it does not necessarily mean that this injury is directly related to the investigational drug administration. Other factors need to be considered. For instance, if the standard of care or alternative treatment is an oral medication, then the i.v. infection may be directly attributed to the investigational study drug. However, if the standard of care treatment is also intravenous, then the infection maybe construed as being a consequence of this typical intravenous procedure, and therefore, not directly related to the investigational drug administration.
When the trial is not conducted pursuant to a private industry sponsor protocol, the costs of treating study subjects for injuries or complications resulting from a study material or research procedures will be the responsibility of the subject or the subject’s Medicare/private insurance plans. Further guidance is available under “Compensation for Injury” in the HRP-109 – Consent Language Document.
It is not uncommon for participants to be paid for their participation in research, especially in the early phases of investigational drug, biologic or device development. Financial remuneration is often used when health benefits to participants are remote or non-existent. Payment to research participants for participation in studies is not considered a benefit, it is a recruitment incentive. However, the payment should appropriate to what is being asked of the participant to do during the study and relative to the potential harm/discomfort of participating in research. The payment should not unduly induce a person to participate.
The Institutional Review Board (IRB) should determine that the risks to participants are reasonable in relation to anticipated benefits [21 CFR 56.111(a)(2)] and that the consent document contains an adequate description of the study procedures [21 CFR 50.25(a)(1)] as well as the risks [21 CFR 50.25(a)(2)] and benefits [21 CFR 50.25(a)(3)]. Therefore, the IRB should review both the amount of payment and the proposed method and timing of disbursement to assure that either are coercive or present undue influence [21 CFR 50.20].
The amount and schedule of all payments should be presented to the IRB at the time of initial review. Any credit for payment should accrue as the study progresses and not be contingent upon the subject completing the entire study. Unless it creates undue inconvenience or a coercive practice, payment to participants who withdraw from the study may be made at the time they would have completed the study (or completed a phase of the study) had they not withdrawn. For example, in a study lasting only a few days, an IRB may find it permissible to allow a single payment date at the end of the study, even to participants who had withdrawn before that date.
While the entire payment should not be contingent upon completion of the entire study, payment of a small proportion as an incentive for completion of the study is acceptable to FDA, providing that such incentive is not coercive. The IRB should determine that the amount paid as a bonus for completion is reasonable and not so large as to unduly induce participants to stay in the study when they would otherwise have withdrawn. All information concerning payment, including the amount and schedule of payment(s), should be set forth in the informed consent document.
For further information, see FDA’s Guidance for Institutional Review Boards and Clinical Investigators; Payment to Research Participants Information Sheet.
Penn State University classifies participant payment into two categories: Stipend and Reimbursement. Stipend is payment for participant’s (and caregiver’s, if applicable) time to participate in research. Stipend can be paid as a flat amount or on a per-hour basis. This type of payment is subject to the U.S. Internal Revenue Service regulations, and may be deemed earned income. Reimbursement is for expenses incurred due to participation in research. Often reimbursement is for travel expenses, thus this category is referred to as “Travel.” Travel expenses can be mileage, gas, tolls, airline or train tickets, cab/rideshare fares, and parking. However, Reimbursement can include, but not limited to, hotel expenses related research visits, meals, and supplies/equipment necessary to participate in the research. Reimbursement can be paid as a flat amount or for actual cost upon submission of receipt.
For details, see Penn State’s research protections guideline Payments to Human Participants in Research (RPG03) .
The Penn State IRB Investigator Manual (HRP-103) provides a wealth of information about the IRB. It is advisable that the investigator consult this document prior to preparing the application. The IRB Investigator Manual is available in the CATS IRB Library. The Penn State IRB is an administrative body established to protect the rights and welfare of human research subjects recruited to participate in research studies conducted under the auspices of Penn State University and Penn State Health. The role of the IRB is to review and to make decisions on all research involving human subjects.
Types of regulatory review for research activities
Submitted research activities may fall into one of the following four regulatory classifications:
- Not “Human Research:” Activities must meet the organizational definition of “Human Research” to fall under IRB oversight. Activities that do not meet this definition are not subject to IRB oversight or review. Review the IRB “WORKSHEET: Human Research Determination (HRP-310)” for reference. Contact the IRB Office in cases where it is unclear whether an activity is Human Research.
- Exempt: Certain categories of Human Research may be exempt from regulation but require IRB review. It is the responsibility of the organization, not the investigator, to determine whether Human Research is exempt from IRB review. Review IRB “WORKSHEET: Exemption Determination (HRP-312)” for reference on the categories of research that may be exempt.
- Review using the Limited IRB Review Procedure: Certain categories of exempt Human Research require that the IRB assess some [46.111(a)(7)], but not all, of the 45 CFR 46.111 review criteria. Review IRB “WORKSHEET: Limited IRB Review (HRP-319)” for reference on the categories of research that may be eligible for a limited IRB review.
- Review Using the Expedited Procedure: Certain categories of non-exempt Human Research may qualify for review using the expedited procedure, meaning that the project may be approved by a single designated IRB reviewer, rather than the convened board. Review IRB “WORKSHEET: Eligibility for Review Using the Expedited Procedure (HRP-313)” for reference on the categories of research that may be reviewed using the expedited procedure.
- Review by the Convened IRB: Non-Exempt Human Research that does not qualify for review using the expedited procedure must be reviewed by the convened IRB.
Criteria for IRB Approval
In order to evaluate and potentially approve human subjects research, the Penn State IRB must review the protocol and determine that all of the federal requirements for approval, as outlined in 45 CFR 46.111(a)(1-7)(b), are satisfied. The criteria for IRB approval can be found in the “WORKSHEET: Criteria for Approval and Additional Considerations (HRP-314)” for non-exempt Human Research. The worksheet references other checklists that might be relevant. All checklists and worksheets can be found in CATS IRB Library.
What are the decisions the IRB can make when reviewing proposed research?
The IRB may approve research, require modifications to the research to secure approval, table research, or disapprove research:
- Approval: Made when all criteria for approval are met. See “Criteria for IRB Approval” above.
- Modifications Required to Secure Approval: Made when IRB members require specific modifications to the research before approval can be finalized.
- Deferred: Made when the IRB determines that the board is unable to approve research and the IRB suggests modifications the might make the research approvable. When making this motion, the IRB describes its reasons for this decision, describes modifications that might make the research approvable, and gives the investigator an opportunity to respond to the IRB in person or in writing.
- Tabled: Made when the IRB cannot approve the research at a meeting for reasons unrelated to the research, such as loss of quorum. When taking this action, the IRB automatically schedules the research for review at the next meeting.
- Disapproval: Made when the IRB determines that it is unable to approve research and the IRB cannot describe modifications the might make the research approvable. When making this motion, the IRB describes its reasons for this decision and gives the investigator an opportunity to respond to the IRB in person or in writing.
The IRB must review and approve all Human Research activities prior to the initiation of any research activities. Create an online application in CATS IRB, and submit it to the IRB along with all required documents. All research submissions must have a protocol attached to the online application in CATS IRB. The purpose of the protocol is to provide the IRB with sufficient information to conduct a substantive review. If a separate sponsor’s protocol exists, please submit it in addition to Local Site Plan for Human Subject Research (HRP-595) (see below). The IRB has provided multiple protocol templates, based on the type of research being conducted. Protocol templates can be accessed by navigating to CATS IRB (login required), clicking on the Library link in the left menu and then clicking on the templates tab on that page. The templates are referenced by number and include:
- For social science/non-biomedical/educational research
- For biomedical research studies not involving the use of a test article (drugs or devices)
- For biomedical research involving the use of a test article (drugs or devices, supplements, alternative medicines and/or chemicals)
- For biomedical research that falls under the FDA regulations
- For studies involving the use of a Humanitarian Use Device (HUD)
- For activities for which the need for IRB approval or determination is unclear, or
- For activities requiring written documentation of a not human research determination
- For multi-center research for which a protocol has been provided by the sponsor or director of the multi-center study. Upload both the sponsor-written protocol and HRP-595 for this type of project
- For studies involving the review of medical records (electronic medical records or paper charts) and/or the analysis of existing restricted data sets only . This protocol is not for the use of data that does not meet the definition of human subject research. Researchers who are not using human subjects data can complete protocol template HRP-594 – Protocol for Not Human Subjects Research Determination, when necessary.
- For all studies reviewed at Penn State College of Medicine, as a supplement to the main protocol document
Use a template protocol as a starting point for drafting a new protocol, and reference the instructions in italic text for information the IRB looks for when reviewing research. Here are some key points to remember when developing a protocol:
- The italicized bullet points included in the gray boxes in the protocol template serve as guidance to investigators when developing a protocol for submission to the IRB. All gray boxes should be left in the final document.
- If the study is a multi-center study, and the sponsor has provided a protocol, upload the sponsor’s protocol in CATS IRB and the Local Site Plan for Human Subjects Research (HRP-595).
- When writing a protocol, always keep an electronic copy. You will need to modify this copy when making changes to the protocol, or if the IRB requests changes.
- Note that, depending on the nature of your research, certain sections of the template may not be applicable to your protocol. Indicate this as appropriate.
- Adults unable to provide legally effective consent
- Individuals who are not yet adults (infants, children, teenagers)
- Pregnant women
- Neonates of uncertain viability or non-viable neonates
- Research studies using a community-based participatory research design
- Use of community advisory boards
- Use of subject advocates
- Partnerships with community-based organizations
- Appropriate human subject training for community partners engaged in the research
Supporting Documents
CATS IRB will prompt the user to upload documents throughout the submission form, including consent forms, protocols, recruitment materials, etc. In addition, any other study-specific documents should be uploaded in the “Local Site Documents” section of the form. Examples of common supporting documents include:
- Surveys/questionnaires
- Interview questions/focus group topics
- Observation checklists
- Videos or images that subjects may be asked to view (stimuli)
- Certificates of confidentiality from HHS Agency
- Scientific Review Memo
- HRP 903 Radiation Review Form – all research involving diagnostic or therapeutic radiation procedures involving ionizing radiation
- HRP 902 Human Tissue for Research Form – used when a project involves collection of tissue for research
- Enterprise Information Management (EIM) Design Specification Forms
- Completed checklist of Department of Energy requirements, if applicable
- Other IRB approvals
- Other study-related documents not previously uploaded
Informed Consent
The IRB has multiple consent templates, based on the type of research being conducted. Consent templates can be accessed by navigating to CATS IRB Library. Use one of the following templates:
- HRP-580 – HRPP Consent Form Template: For studies obtaining written informed consent (This is the standard long form consent document discussed in the next section)
- HRP-581 – HRPP Consent Form Addendum: To inform current subjects about new information that could affect the subject’s willingness to continue in the study
- HRP-582 – HRPP Consent Form for Emergency Use: To obtain written informed consent from patients receiving an unapproved drug, biologic or device in an emergency situation
- HRP-583 – HRPP Consent Short Form: Use this template for the short form consent documentation
- HRP-584 – HRPP Consent Guidance for Exempt Research: For the consent process in research projects that are exempt and involve interactions with research subjects
- HRP-585 – HRPP Minimal Risk Consent Form Template: For research in which verbal or implied consent will be obtained and which will not involve the use of protected health information
- HRP-586 – HRPP Pregnant Partner Consent Form: For research in which the partner of a subject becomes pregnant during participation in a clinical trial involving investigational drug(s). Note: this document does not have to be submitted with the initial study review but should be used if/when it becomes applicable.
Note that all long form consent documents and all summaries for short form consent documents must contain all of the required and all additional appropriate elements of informed consent disclosure. Review the “Long Form of Consent Documentation” section in the IRB’s “WORKSHEET: Criteria for Approval (HRP-314),” to ensure that these elements are addressed. The IRB requires that you date the revisions of your consent documents in the header to ensure that you use the most recent version approved by the IRB. The approved version will be watermarked by CATS IRB.
Common Mistakes in Informed Consent
- Incomplete and/or inconsistent information
- Language is too complex
- Recruitment and consent process is not well explained
- “De-identified” not a meaningful term by itself
- Standard of care procedures vs. research procedures are not clearly described
- Use of exculpatory language
Helpful Hints: Consent versus Clinical Trial Agreement (Contract)
The Consent Form
- Is not a contract for exchange of services for payment, but an acknowledgement
- It is between Penn State and the participant
- Necessary for regulatory compliance purposes
- Project-specific
The Clinical Trial Agreement (Contract)
- Is a contract for services by PSU/PSH in exchange for payment: required only when we are being paid by a Sponsor to conduct a trial
- It is between PSU/PSH and the Sponsor (the PI and the study subjects are not parties to the contract)
- It is necessary to cover the legal risks between the parties in exchanging services for payment
- May be a template or master and not project-specific
Approvals required prior to initiating research
Anatomic Pathology
Research involving the collection of tissues or use of pathologic specimens must receive approval from Anatomic Pathology. Include a copy of the Use of Human Tissue for Research Form with the application materials for research projects that involve any of the following:
- Collection of tissue from surgical or biopsy procedures;
- collection of bone marrow from bone marrow biopsies and/or bone marrow aspirates;
- use of archival pathologic specimens stored in Anatomic Pathology;
- collection of tissue from cadavers; and/or
- collection of placenta specimens.
There is a charge per specimen to help defray the technical cost in obtaining research tissues. Call the Department of Pathology at 717-531-8352 , for the current charge to use when preparing the budget for a grant. If the investigator is aware that a biopsy or excision of the desired tissue is scheduled, they should complete and submit to the Anatomic Pathology Gross Room (fax to 717-531-0831) a “Research Tissue Request Form” prior to the scheduled date of surgery. This form notifies the Gross Room staff that a specimen is a potential source of research tissue so that the specimen can be handled appropriately for that purpose. A blank copy of this form can be obtained from the Gross Room; this blank form can be photocopied for multiple submissions. Anatomic Pathology approval is required before the IRB will approve the study.
Human Use of Isotope Committee
All research involving radiation procedures (standard of care and/or research-related) must complete the Radiation Review Form and upload it on the Supporting Documents page in the CATS IRB application. If the study involves use of radiation procedures for research purposes, the study must receive approval from the Radiation Safety Committee – Human Use of Isotope Committee (HUIC). HUIC approval is required before the IRB will approve the study.
Clinical Research Center Advisory Committee
Research involving the use of services at the Clinical Research Center (CRC) for any reason, including the use of personnel as back up to the research team or plans to use personnel in the event of an emergency, need to be reviewed by the CRC Advisory Committee. CRC Advisory Committee approval is not required before the IRB will approve the study.
Conflict of Interest Committee (Individual)
Research studies in which a member of the study staff has a financial interest as defined by PSU policies must be reviewed by the Conflict of Interest Review Committee (CIRC-HY). IRB approval will not be granted until the IRB has reviewed the recommended management plan.
Conflict of Interest Committee (Institutional)
Research studies in which an institutional conflict may exist as defined by Penn State policies must be reviewed by the Institutional Conflict of Interest Review Committee. IRB approval will not be granted until the IRB has reviewed the recommended management plan.
Departmental Scientific and Feasibility Review
All investigator-written research studies requiring review by the convened IRB must provide documentation of scientific review with the IRB submission. The scientific review requirement may be fulfilled by one of the following:
- external peer-review process (e.g., research studies funded by an NIH grant);
- departmental or institute scientific review committees; or
- scientific review by the Clinical Research Center Advisory Committee.
All research studies involving cancer patients, records and/or tissues or cancer prevention studies must be reviewed by the Penn State Cancer Institute Scientific Review Committee.
Institutional Animal Care and Use Committee
Research involving vertebrate animals must receive approval from the Institutional Animal Care and Use Committee. Investigators will need to complete an IACUC application. The IACUC approval is required before IRB approval will be issued.
Data Transfer Agreement Review
Research that involves any transfer of human research data to and/or from any third party requires review by the Office of Research Affairs. This approval is required before IRB approval will be issued. Requests for Data Use Agreements should be submitted via this form .
Data Security Plan Ancillary Review
Research in which the data security plan does not meet the requirements of the SOP Addendum – Security and Integrity of Human Research Data require review by the IT Security Group at Penn State Health. Also, research that involves the transfer of PHI or PII to and/or from a third party (exceptions: industry-sponsored, multi-center trials, oncology group studies and studies sharing data with NIH genomic databases) require review by the IT Security Group. The IT Security Group approval is required before IRB approval will be issued.
Medical Education Ancillary Review
Educational research enrolling any of the learner groups at Penn State College of Medicine must be reviewed by the Educational Research Review Committee (ERRC). The ERRC review must be complete before IRB approval will be issued.
IND/IDE Audit Ancillary Review
Research in which a Penn State Health or Penn State College of Medicine researcher holds the IND or IDE or intends to hold the IND or IDE must be reviewed by the Research Quality Assurance Office (RQA) to ensure sponsor-investigator is compliant with FDA sponsor requirements (including GMP when applicable). Also, RQA may be asked to review investigator-written research using marketed drugs in which the researcher does not have an IND and there is no exemption determination from the FDA. The RQA review must be completed before IRB approval will be issued.
Institutional Biosafety Committee
Research involving biohazardous materials (human biological specimens, biological toxins, carcinogens, infectious agents, recombinant viruses or DNA or gene therapy) must receive approval from the Institutional Biosafety Committee. Investigators will need to complete an IBC application. The IBC approval is required before IRB approval will be issued.
Biological Use Authorization
Institutional Biosafety Committee (IBC) at The Pennsylvania State University College of Medicine reviews research activities involving biological materials that may pose a risk to human, animal, or environmental health. IBC review and approval is required for research activities involving use of recombinant or synthetic nucleic acids, potential employee exposure to infectious agents, and generation of medical waste outside of clinical (patient care) environments.
Examples of research activities that require IBC review and approval include:
- Including use of human blood, tissues and patient samples for research purposes used in clinical trials
- Primary or established human-derived cell lines
- Biological toxins or carcinogens
- Infectious agents
- Recombinant infectious agents
- Recombinant DNA or synthetic DNA molecules
- Recombinant DNA in animals
- Biohazards in humans
- Biohazards in animals
- Genetically manipulated in animals
- Employee exposure to any of the aforementioned activities or materials, including product manipulation, pack, transporting and shipping
Researchers who plan on conducting research involving use of experimental recombinant or synthetic nucleic acid technologies in human subjects including all human gene transfer research are required to contact the Biosafety Office at the earliest stages of their research plan for advising by emailing [email protected] .
Penn State uses the CATS Safety electronic protocol submission system for all renewal and new submissions.
Approved protocols are valid for 3 years, after which the protocol must be resubmitted with any modifications for a full review by the IBC.
Principal Investigators must submit the protocol through the CATS Safety system at researchsafety.psu.edu , prior to submission of 1) the IAF for a grant application to the Office of Research Affairs, 2) animal protocols that involve work with biohazards to the Institutional Animal Care and Use Committee (IACUC), or 3) protocols that involve work with biohazards to the Institutional Review Board (IRB).
In addition, investigators must submit through the CATS safety system when beginning work with a new rDNA source (organism), new sequence (gene or gene region), recipient cell type for rDNA (host), or vector (plasmid or virus). All changes to a protocol approved are to be amended at anytime to update work, add additional clinical trials, training and personnel updates appropriate.
CATS Safety includes an extensive reference section under the CATS Library button. Information includes the appropriate risk group summary, templates, policies , required training matrix and additional biosafety resources.
Activities conducted in clinical environments that are not used for research purposes, by employees covered under established health surveillance and infection control plans do not generally require IBC review.
The Principal investigator (PI) is responsible for completing all required training and for ensuring their employees complete required training commensurate with tasks performed. Required trainings include
- Blood Borne Pathogen (BBP) or Annual infection control training
- Annual Safety Training – Infonet (Penn State Health ePass login required)
- Biological Shipping and Dry Ice training – Infonet (Penn State Health ePass login required)
Questions regarding the IBC review process or training schedules and requirements should be directed to the Biosafety Officer at [email protected] .
Biosafety at Penn State College of Medicine is based on the two primary documents listed below
- The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines)
- Biosafety in Microbiological and Biomedical Laboratories (BMBL; sixth edition)
- Penn State Policy RP11 Use of Regulated and Biohazardous Materials in Research and Instruction (formerly SY24)
Stem Cell Research Oversight Committee
The Institutional Biosafety Committee (IBC) provides administrative support on issues involving stem cell research.
Principle Investigators must add all appropriate information in the CATS safety submission regarding Human Embryonic Stem cell or pluripotent stem cell research.
- Human Embryonic Stem Cell (hESC) and/or Human Induced Pluripotent Stem Cell (hiPSC) Research must also be submitted in the CATS Safety protocol submission system .
- The submission will be reviewed by the Penn State ESCRO committee for formal review and approval.
All research laboratory information is available on the internal informational database, LabManager . As of Jan. 1, 2023, the LabManager system is being upgraded and only available to the Research Quality Assurance office
Technology Evaluation Process
Clinical research that requires implementation or use of new applications, systems or devices requires an IT Evaluation prior to the start of the study. This is necessary to determine whether or not the new technology may inadvertently create technical instability or create security risks for the institution. Some examples of research-related technologies that have been evaluated include:
- Mobile devices that are used to gather research data on human subjects
- Software that would be implemented on the Penn State network or that would be used to access the Penn State network
- Clinical devices that require network access or involve data transfer
It is important to note that an IT evaluation is restricted to a review of the technical stability, security and feasibility of technology used at the health system. Approval of applications, systems or devices does not signify a commitment of IT resources, implementation costs or financial approval. Learn more on the IT support section of the Infonet (internal access only; login required).
Submitting to IRB and Obtaining IRB Approval
The Human Research Protection Program (HRPP) accepts electronic submissions through CATS IRB, which is accessible at irb.psu.edu . The section of this guidebook on How to Submit a New Human Research Study to the IRB provides additional details regarding the submission of forms and documents through CATS IRB.
To access CATS IRB, you must first have a Penn State Access Account. CATS IRB access is further limited to individuals with authorized CATS IRB user accounts. If, after WebAccess authentication, you receive the message “We are unable to display the requested page due to a problem verifying your authentication information,” you must request access to CATS IRB by emailing [email protected] .
In addition, all Penn State faculty, staff and students will be required to enroll in and use Microsoft Authenticator (Penn State’s multifactor authentication) as the identify-verification method for accessing secure Penn State resources. For more information, visit Penn State’s Multifactor Authentication (MFA) Setting website. The IRB Researcher’s Guide , available in the CATS IRB Help Center, provides a step-by-step guide for the study staff for creating and submitting a study, responding to clarification requests, and getting started with modifications, continuing reviews, and new information reports.
The IRB will provide you with a written decision indicating the IRB’s determination.
- If the IRB has approved the human research: The human research may commence once all other institutional approvals have been met. IRB approval is active for a limited period of time which is noted in the approval letter.
- If the IRB requires modifications to secure approval and you accept the modifications: Make the requested modifications and submit them to the IRB. If all requested modifications are made, the IRB will issue a final approval. Research cannot commence until this final approval is received. If you do not accept the modifications, write up your response addressing the specific modification(s) that are in question and submit it to the IRB.
- If the IRB defers the human research: The IRB will provide a statement of the reasons for deferral and suggestions to make the study approvable and give you an opportunity to respond in writing. In most cases if the IRB’s reasons for the deferral are addressed in a modification, the human research can be approved.
- If the IRB disapproves the human research: The IRB will provide a statement of the reasons for disapproval and give you an opportunity to respond in writing.
In all cases, you have the right to request that the IRB reconsider a decision by submitting a written response to the IRB in the CATS IRB system. If the IRB has disapproved the study or submission, new information that was not previously provided to the IRB must be provided for consideration by the IRB.
See the section of this guidebook on documentation maintenance for details.
Submitting Contracts for Approval
The Office of Research Affairs (ORA) at Penn State College of Medicine oversees the proposal submission process and negotiates contractual terms and conditions of awards, all with the goal of promoting, fostering and sustaining excellence in basic and clinical research.
In collaboration with other offices, we strive to provide leadership which promotes the protection of human subject volunteers, the safety of research personnel, the humane treatment of research animals, the stewardship of research funds, the highest standards of ethics, integrity, and objectivity in the research process.
See details about the Office of Research Affairs .
ORA Receipt and Assignment
Once the contract packet is received by the contracts office it is assigned to a contract administrator contract administrator for review, negotiation and final execution. The contract administrator contract administrator will work with the Clinical Trials Office (CTO), and department contact if there are missing elements or delays with negotiations.
ORA Initial Review
The contract administrator contract administrator will review the contract for consistency with university policy, state and federal law, using the budget, protocol and internal forms as necessary. The contract administrator may also seek consultation with Risk Management, Legal, IRB, CTO, or other sources as necessary to complete the initial review.
ORA First Comments and negotiation to Sponsor
The contract administrator will send a marked copy of the agreement to the sponsor. The contract administrator will provide reasonable updates on the agreement to the study team.
End of Negotiation
Once there is agreement between the contract administrator and the sponsor, the contract administrator and the CTO perform final congruence between the agreement, internal and sponsor budgets and informed consent.
Once approved, the contract will be sent for signature, typically starting with the PI signature of acknowledgement to the contract. After the PI signs the contract, the authorized signatories of Penn State College of Medicine and/or Penn State Milton S. Hershey Medical Center or applicable Penn State Health entity sign the agreement on behalf of the institutions. Typically the Sponsor signs last and the agreement is then fully executed. However, the contract will not be awarded until the IRB has approved the project.
After the fully executed agreement is returned to ORA, and the IRB has approved the protocol, the ORA notifies the post-award central finance administrators. The post-award central finance administrators open the extramural account.
After the contract is executed, ORA and CTO will be responsible, upon request from the study team, to negotiate and execute amendments to the project period, budget, or other required changes to the contract as agreed between the sponsor and the institution. Such requests must originate from the study team, rather than directly from the sponsor.
Study Activation
For industry-sponsored and federal flow-through studies: Once the study is approved in the Internal Approval Form (IAF) and the Statement of Award (SOA) is issued internally, the IAF information is transmitted over into a form inSIMBA. SIMBAis the accounting and form processing system at Penn State College of Medicine. A notice is sent out to the financial contact for the study and the contact goes into SIMBA to enter information into various fields. The form is sent for final approval by University Park Research accounting. They return the form with a cost center and internal order.
The study team uses the cost center and internal order in order to apply expenses to the clinical research study. The financial contact allocates the total statement of award into different general ledgers. The Controller’s Office ensures that expenses and income falls within these different categories. These categories are established by the contract and budget with the sponsor. The amounts are negotiated by the pre-award analyst. The income and expense are reconciled by the post-award analyst.
The cost center and internal order is entered into CATS IRB so that the IRB can bill the appropriate study. This information is also entered into the Hospital Finance database so that study related charges can be applied to the account. Investigator effort is applied by adding this information to the salary assignment and allocated based on study activity. Study team effort is applied in a similar fashion depending if they are College of Medicine employees or Penn State Health employees.
The Coverage Analysis Review (CAR) memo and billing grid (BGRID) created by the College of Medicine Clinical Trials Office (CTO) will be the sole source of truth for determining the responsible payer for Penn State Health (PSH) billable items and services.
Study Tracking and Analysis for Research (STAR) is the sole clinical trial management system to be used for tracking PSH billable items and services for research participant’s visits.
The information entered into STAR will allow Patient Financial Services (PFS) to direct charges to the appropriate payer. On a monthly basis, reports are generated by PSH Finance and verified by the study team in order to bill the research services to the study.
See the Clinical Research Monthly Procedure Reports section of this guidebook for details.
The ClinicalTrials.gov Protocol Registration and Results System (PRS) is a web-based tool used to submit clinical study information to ClinicalTrials.gov. Records submitted through the PRS are available to the public at ClinicalTrials.gov. Contact RQA at [email protected] for PRS account acquisition and any additional guidance or questions. Also see the User Guide (internal access only; login required) to ClinicalTrials.gov Registration at Hershey. ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. Title VIII of FDAAA, Public Law 110-85, amended the PHS Act by adding new section 402(j), 42 U.S.C. § 282(j). The new provisions require that additional information be submitted to ClinicalTrials.gov established by the National Institutes of Health (NIH)/National Library of Medicine (NLM). This includes expanded information on clinical trials and information regarding the results of clinical trials. This ClinicalTrials.gov registration requirement applies to:
- Any study initiated by an investigator under IND/IDE: The Sponsor-Investigator submits a certification (FDA Form 3674) attesting that the registration data will be submitted as per regulations. Results reporting would also be required for this type of study, due within one year of the study end date. Single-patient, emergency-use INDs do not fall under the referenced section, and therefore are not required to submit certification. Any study not conducted under an IND/IDE but involving drug or device. Most Phase 1 trials are not required to register.
- NIH-funded interventional clinical trials.
- Studies that intend to publish in scientific peer-reviewed journals need to be registered and results entered into ClinicalTrials.gov. Investigators intending to publish clinical studies results in an ICMJE journal (International Committee of Medical Journal Editors) must register before enrollment of first subject. The ICMJE clinical trial registration policy requires prospective registration of all interventional clinical studies, but does not require r results reporting for registered trials. In June 2007, the ICMJE adopted the WHO’s definition of clinical trial. Learn more about the definition and ICMJE publication on the ICMJE Clinical Trials Registration FAQs page and the ICMJE Publishing and Editorial Issues: Clinical Trial Registration page.
Potential Consequences of Non-Compliance
- Civil or criminal judicial actions
- Monetary penalties up to $11,383 per day
- Loss of current or future funding
- Rejection for manuscript publication for ICMJE journals
In 2013, the Centers for Medicare and Medicaid Services (CMS) issued a Transmittal requiring new mandatory reporting of the ClinicalTrials.gov clinical trial number (also known as NCT number) on all hospital and professional claims for related items/services. Effective Jan. 1, 2015, it became mandatory to report the clinical trial number on claims for items/services provided in all clinical trials that are qualified for coverage. In order for the NCT number to correctly appear on the claims, the study teams need to provide the NCT number in CATS IRB.
FDA Webinar Series – Overview of ClinicalTrials.gov
In a three-part webinar series, FDA provides a general overview of ClinicalTrials.gov and relevant definitions, laws, and regulations for complying with ClinicalTrials.gov registration and results information submission requirements. Participants will gain an understanding of CDER’s role and responsibilities with respect to ClinicalTrials.gov oversight and will hear examples of compliance and enforcement activities CDER has taken to encourage compliance.
Access the webinar series here
Other Relevant Links
- NIH Guidance on ClinicalTrials.gov Registration Requirements
- ClinicalTrials.gov Protocol Registration System
Clinical Engineering is primarily responsible for servicing and supporting technology used in the direct care and treatment of patients. The department also supports some minor clinical laboratory equipment and various devices used in the College of Medicine. The overall scope and responsibility of the Clinical Engineering Department extends into Penn State Health’s various off-campus practice sites including, but not limited to, Nyes Road, Elizabethtown, Palmyra, Fishburn Road, Front Street, Middletown, Erford Road, Lancaster, Silver Springs, State College and Wilkes-Barre. FDA-approved equipment used on human patients at Penn State Health requires evaluation and testing by Clinical Engineering. For instance, blood pressure monitors and EKG machines sent by an industry sponsor to support multicenter trials need to be evaluated. Another example would be if a researcher was studying (under an IRB-approved protocol) not-yet-approved ultrasound contrast material provided by a pharmaceutical company, while also using the pharmaceutical-company-owned, commercially available, ultrasound machine, the ultrasound machine would need to be checked by Clinical Engineering. Studies with medical devices that have not yet been FDA approved require an IRB approval, and the evidence of the IRB approval should be provided to Clinical Engineering. For more information, see the Clinical Engineering Infonet section (internal access only; login required) or call 717-531-8410 .
Institutional Policies and Procedures
- DE-06SPM, Medical Equipment Management Plan (login required)
- A-09 HAM, Patient Safety Event Reporting (login required)
- A-70 HAM, Failure of Medical Product or Device/Equipment (login required)
See the Maintain Study Documentation section of this guidebook for details.
Subject Recruitment
When an established patient is being considered for participation in a research study by a clinician involved in the patient’s care, the HIPAA rules can be confusing. HIPAA applies when a provider is reviewing a patient’s medical record for both treatment and research purposes. In general, under the HIPAA privacy rules, patient’s medical information may be accessed for treatment, payment or operational purpose without obtaining prior written authorization. Access to a patient’s medical record for any other purposes may require additional steps to be in compliance with privacy laws and rules. This means that when a provider looks at his or her patient’s medical record for research purposes, the research-related HIPAA rules apply.
If a patient’s record is reviewed for a treatment purpose ( e.g. , to view lab results or consult with a referring provider) the research-related rules do not apply. However, once a patient’s medical information is viewed for a research-related activity ( e.g. , to screen for eligibility or review, to review a unique case for possible study, or to collect data) the research-related HIPAA rules apply. For example, if a provider is reviewing a patient’s lab report for regular care, this access would be for treatment purposes and the research-related rules would not apply. However, if during this review, the provider notices that the lab value may make them a potential research subject and wants to review the chart further for eligibility; the research-related rules would need to be considered.
In general, before any patient information can be used for a research purpose, the patient must sign and date a study-specific consent form that includes the HIPAA authorization elements or a separate HIPAA authorization form which recites the patient’s privacy rights. This is true whether or not the patient is seen by the researcher/physician for medical care. Patient information cannot be used for research-related purposes without a signed patient authorization. There are two limited exceptions: if the IRB has granted a Waiver of Authorization or if the IRB has granted a “Preparatory to Research Authorization.” Important: Any study data obtained without the proper authorizations cited above may not be used for publication (i.e. journals, abstracts, etc.) or any other purpose and can be subject to notification requirements under state and/or federal laws.
See the section of this guidebook titled “Assessment of Potential Cohort for Feasibility or Recruitment” for details.
A HIPAA Waiver of Authorization can be obtained from the IRB if access to patient data is needed for recruitment purposes.
Describe the need in the “HIPAA Research Authorization and/or Waiver or Alteration of Authorization” section of the protocol template or the protocol site addendum. This section is reviewed by the IRB.
If a partial waiver of authorization for recruitment is granted, access to identifiable patient data to determine if a patient may be a potential research subject is permitted.
IRB approval is confirmed by issuance of the IRB approval memo for the study.
The requirement to obtain authorization may be waived or altered if certain criteria are met. Refer to “CHECKLIST: HIPAA Waiver of Authorization (HRP-441)” in the CATS IRB Library for a list of the criteria.
Authorization may be waived for all, or only some uses of protected health information (PHI) for a particular study. A partial waiver permits the use of PHI for recruitment purposes only, to allow identification and, as appropriate, contact of potential participants to determine their interest in study participation. The requirement to obtain authorization for use of PHI may also be altered or waived for a specific study. An alteration allows a change in certain authorization requirements, while still requiring authorization for the use of PHI. Examples include making an exception to the required language in an authorization or to the requirement to obtain a signed authorization. An alteration must meet the same criteria as a waiver or partial waiver.
To request a partial waiver of authorization for recruitment, you must complete the “HIPAA Research Authorization and/or Waiver or Alteration of Authorization” section in the protocol or protocol site addendum for the study. Appendix A-11 of the IRB Investigator Manual includes a list of informational elements that are considered to be identifiers according to the HIPAA regulations.
For additional information see Penn State Policy RP07 – HIPAA and Research at Penn State University .
An application for the use of PHI from decedents must be submitted to the HRPP prior to engaging in the research. In order to gain access to the PHI, the principal investigator needs to demonstrate that the use or disclosure being sought is solely for research on the PHI of decedents, adequate documentation of the death of such individuals, and that the PHI for which use or disclosure is sought is necessary for the purposes of the proposed research. Submit the Request for Research on Decedents’ Information Attestation Form . If the research will include any identifiers linked to living persons, the project must be approved by the IRB in advance. For more information about the Privacy Rule and decedent research provisions go to: 45 CFR 160.103, paragraph (2)(iv) of the definition of “protected health information.”
Because it may be necessary for a researcher to obtain access to and review PHI in order to prepare a research study, HIPAA rules allow such a review upon compliance with specified criteria. Prior IRB approval must be obtained before accessing and reviewing PHI to prepare a study. Submit the Review Preparatory to Research Request located under “Forms” for IRB for review and approval.
HIPAA rules require that a record be made of a disclosure of any personally identifiable information that is made without an authorization by the research participant. Therefore, tracking of disclosures will have to be undertaken for all disclosures if a waiver of authorization, an approval for review preparatory to research or an approval for the use of a decedent’s PHI is obtained for purposes of research, and for any disclosures not previously specified in a signed authorization document. For purposes of this policy, “disclosure” means the release, transfer, provision of access to, or divulging in any other manner of PHI to any person, whether or not employed by Penn State Health, Penn State College of Medicine or Penn State University, who is not participating in carrying out the research protocol. The following information about any disclosure must be recorded and made available to the individual who is the subject of the PHI upon request:
- Date of disclosure;
- Name of person/entity that received the PHI;
- Description of what PHI was disclosed;
- Brief statement regarding the purpose of the disclosure.
If a research protocol requires multiple disclosures to the same outside party over a period of time, the following information is adequate:
- For the first disclosure, all of the above must be recorded;
- For subsequent disclosures, tracking can refer to the initial record of disclosure and should include the frequency, periodicity or the number of disclosures that will be made; and
- The date of the last disclosure must be documented.
Large Studies: When tracking is required and involves the disclosure of PHI from more than 50 people, HIPAA rules allow a modified tracking method. In this instance it is unnecessary to maintain a list of the specific persons about whom PHI has been disclosed, but the following information must be available upon the request of any individual whose information may have been included:
- The name and description of all protocols involving 50 or more people for which authorization has been waived, including the purpose of these and criteria for selecting records;
- Brief descriptions of types of PHI disclosed;
- Dates or time periods during which disclosures occurred;
- Contact information (name, address, telephone number) for sponsors and recipient researchers; and
- Statement that a specific individual’s PHI may or may not have been disclosed for a particular protocol or research activity.
In addition, the researcher must also assist in contacting the sponsor and recipient researcher if it is reasonably likely that an individual’s PHI was disclosed to them. Tracking information as required by HIPAA rules must be maintained by the principal investigator at least six years, and made available to the Privacy Officer.
The IRB must review and approve all materials for human subject recruitment before recruitment efforts begin. An advertisement to recruit subjects is any form of materials whose main purpose is to inform and invite the potential subjects to participate in a research study, including:
- Flyers and handouts
- Letters and emails
- Newspaper or magazine ads
- Radio, TV and cable
- Internet postings
- Phone scripts
- Facebook and other social media
- Oral communication
The advertisement should be limited to the information prospective subjects need to determine their eligibility and interest, such as:
- Name and address of the investigator or research facility;
- The condition under study or purpose of the research;
- In summary form, the criteria that will be used to determine eligibility for the study;
- A brief list of participation benefits, if any;
- The time or other commitment required of all subjects;
- The location of the research and the phone number of the person or office to contact for further information.
For FDA-regulated research, the advertisement should not:
- Make claims, either explicitly or implicitly, that the drug, biologic or device is safe or effective for the purposes under investigation.
- Make claims, either explicitly or implicitly, that the test article is known to be equivalent of superior to any other drug, biologic or device.
- Use terms such as “new treatment,” “new medication” or “new drug” without explaining that the test article is investigational.
- Include a coupon good for a discount on the purchase price of the product once it has been approved for marketing.
- State or imply a certainty of favorable outcome or other benefits beyond what is outlined in the consent document and the protocol.
- Promise “free treatment” when the intent is only to say subjects will not be charged for a taking part in the research.
- Include exculpatory language.
- Emphasize the payment or the amount to be paid, by such means as larger or bold type.
Please reference “HRP-315 – Worksheet – Advertisements” in the CATS IRB Library for the IRB’s requirements regarding advertisements meant to be seen or heard by subjects. StudyFinder is also available to enhance recruitment efforts. StudyFinder is a web-based recruitment tool for Penn State researchers, managed and sponsored by Penn State Clinical Translational Science Institute (CTSI). It is available to all Penn State researchers actively recruiting participants or volunteers for studies. StudyFinder displays data in a way that is intuitive and user-friendly for the public. Learn more about the process for listing studies on StudyFinder .
Subject screening is the term used to describe research activities performed on participants after obtaining their informed consent. Usually screening activities are performed to ensure subjects are eligible to be enrolled in the study, i.e. that the participant meets the inclusion and exclusion criteria for the study. Screening activities include interactions with potential subjects to determine eligibility that would not otherwise have been performed if not for the study. Note that a screen failure is the term used to describe the circumstance in which a subject who has provided consent has subsequently failed to meet eligibility criteria for participation in the study based on screening procedures performed after informed consent was obtained. If some or all of the screening activities will take place before signing the consent form ( i.e. , by telephone) the screening script has to be approved by the IRB.
Note that a screen failure is the term used to describe the circumstance in which a subject who has provided consent has subsequently failed to meet eligibility criteria for participation in the study based on screening procedures performed after informed consent was obtained. The IRB does consider subjects where informed consent was obtained, but subsequently failed to meet eligibility criteria for participation, enrolled subjects.
Please reference “HRP-585 – HRPP Screening Procedure Consent Form Template” – For studies obtaining consent for procedures that will occur prior to obtaining consent for the main research project.
If the health information is collected (including verbal responses)from a covered entity, by accessing records or stored identifiable biospecimens, and will be recorded or stored, the investigator must obtain an authorization via a HIPAA Authorization Form. This is required from all subjects in research prior to the use of the disclosure of protected health information (PHI) for any research related purposes. Please reference “HRP-587 – HRPP HIPAA Authorization for Research”.
Please reference the following documents in the CATS IRB Library for the IRB’s policies regarding the informed consent process and the written documentation of consent:
- HRP-090 – SOP – Informed Consent Process for Research
- HRP-091 – SOP – Written Documentation of Consent
For additional information on Assent, Telephone/Remote Consent Process and the Short Form Process, see “HRP-103 – Investigator Manual” – This document is available to guide researchers through policies and procedures related to the conduct of Human Research at Penn State. The IRB has multiple consent templates, based on the type of research being conducted. Consent templates can be accessed in the CATS IRB Library. The CATS IRB Library can be accessed by navigating to CATS IRB , clicking on the Library link in the left menu, and then clicking on the “Templates” tab.
For all research participants who are also Penn State Health patients:
A copy of the signed informed consent form is to be included in the participant’s electronic medical records for all clinical research (regardless if the study meets the NIH definition of a clinical trial. A copy of the signed consent form is to be sent by inter-office mail to Health Information Services.
Documentation of consent is also to be included in the electronic medical record.
See institutional Clinical Research Standard Operating Procedure 401 for further details, which can be found on the Penn State Health Policy Management Portal (ePass login required).
Scheduling and Registration
Schedule training and optimization through CareConnect at [email protected] .
A patient should be associated with the study after signing the Informed Consent utilizing the Alerts Tab in PowerChart. The initial signed Consent Form and all Consent revisions (if a patient was re-consented), must be uploaded into the EMR for each patent enrolled in the study.
Health Information Services is responsible for the consent upload.
The signed paper copies may be sent to HIS by interoffice mail (see the “Submit Copy of Consent to Electronic Medical Record” section of this guidebook for details).
A member of the clinical trial research team is responsible for entering human subject participant information into the PowerChart “Alerts Tab” for all ongoing investigational drug or device clinical trials.
All new order sets or revisions to existing order sets in Connected must now be submitted by completing a form.
The form is available via the Clinical Trials Office .
The study coordinator, or another designated study team member, will receive a monthly report from Penn State Health (PSH) Finance summarizing the charges appearing in the billing system as research related charges for a designated study, including the amount being billed to the College of Medicine.
Study teams should expect to see all charges within two months of the items and services being rendered. If charges have not been listed, the study team is responsible for contacting PSH Finance to investigate further. If STAR entries are incorrect or not completed in a timely manner, misbilling can occur.
Charge reports are correct
Once the review of the report is complete, sign the report where indicated and return to the attention of PSH Finance by the due date. Send a copy to the appropriate central Financial Analyst as well. If clinical research monthly procedure reports are not signed and returned to PSH Finance within three months, the research charges will be deemed correct and billed to the respective study budget. PSH Finance will not adjust any charges after this time.
Charge reports need to revision
If there are services listed on the report that are not research related or not priced correctly, the study team member must notify PSH Finance immediately to have them corrected. Once the report is corrected, a revised copy will be sent for signature.
Revise/amend previous charge reports
Any changes requested to previously billed charges will only be made within three months of the original charge(s) in question. Changes after three months is at the sole discretion of PSH Finance.
Further information
See policy “Use of Clinical Research Funds” (PSHAM F-17) for further details, which can be found on the Penn State Health Policy Manager (ePass login required).
Investigational Drug Pharmacy (IDS)
An investigational drug is defined as a new drug or biological that is used in a clinical investigation and which has not been approved for general use by the U.S. Food and Drug Administration. It may also be a drug which is FDA-approved and is being used in a clinical investigation, possibly outside the use of the FDA-approved labeling. The FDA requires that the investigator or designee establish a record of receipt, storage, use/dispensation, and disposition of investigational drugs. At Penn State Health Milton S. Hershey Medical Center and Penn State College of Medicine, all investigational drugs are handled by the Investigational Drug Service (IDS) Pharmacy, a division of the Department of Pharmacy. IDS manages the receipt, storage, dispensation, return and disposal of study drugs in accordance with Good Clinical Practice Guidelines, the study protocol requirements, and all applicable rules and regulations.
Shipping address: Investigational Drug Service Pharmacy Penn State Health Milton S. Hershey Medical Center 500 University Dr. Room PG200, MC CH79 Hershey, PA 17033 Phone: 717-531-4976 Fax: 717-531-5705 Email: [email protected]
Contact IDS
- Site selection visit
- Budget estimate
- PI-initiated study consult
IRB Application
Setup study.
- Site initiation visit
- Create order template
- Order/receive study drug
- Build computer codes
- Randomization
- Drug compounding
- Staff training
Activate Study
- Order processing
- Drug preparation
- Drug accountability
- Monitor visit(s)
Close Study
- Drug return/destruction
- Close-out visit
- Study archive
The principal investigator instructs the sponsor to ship the study medication directly to the IDS Pharmacy. Upon receipt of study shipments, an IDS staff member will inventory/check the shipment using the shipping invoice, notating lot number, expiration date, breakage, storage condition, and total quantities. The shipping invoice will be signed and dated. The shipment will be recorded on the drug accountability log specific for that study. If applicable, the shipment will be activated in the Interactive Voice/Web Response system and/or the packing list will be faxed to the sponsor. The shipping invoice will be filed in the shipping file for that specific study. The study medication will be placed into the appropriate storage conditions, as designated by the sponsor.
Investigational drugs will be stored separately from other hospital medications, and will be marked (at a minimum) with the drug name, study short name, and IRB number. Dedicated investigational drug refrigerators and freezers will be utilized to store refrigerated and frozen investigational drugs. The outlets for the refrigerators and freezers are connected to the back-up generator. Minimum/maximum temperature logs or continuous electronic temperature monitoring logs for all storage conditions will be maintained daily. IDS will only utilize the institution’s approved, calibrated temperature monitoring device/system. Sponsor provided temperature monitoring devices will not be utilized to record temperature for specific studies during storage on site.
A perpetual inventory will be maintained for every study, to include drug receipt, dispensation, return, and destruction. The IDS pharmacy will not maintain drug accountability records for standard of care medications that are not supplied for the study. The inventory will be audited by the IDS Clinical Trials Assistant or designee. An appropriate minimum inventory will be maintained, based upon the rate of patient enrollment. At the end of a study, the perpetual inventory will be “zeroed out” and the drug will be disposed of/mailed back to the sponsor upon sponsor approval (refer to section 13.3 for additional information pertaining to study closure).
The pharmacy may dispense study drugs supplied for clinical trials only upon the receipt of a written physician order sheet or outpatient prescription signed by a physician-investigator authorized in the state of Pennsylvania to prescribe study drugs, as included on his/her clinical practice agreement. The ordering physician must be included on the study’s 1572 form as an investigator or co-investigator. In order for the drug to be dispensed, the prescription/physician order must contain ALL information required by state and federal law as well as the following information:
- Patient’s name
- Patient’s allergies
- Medical record number
- Protocol name
- IRB and/or PSHCI number
- Schedule of administration
- Quantity to dispense
- Physician name
- Physician signature
The initial order must also contain verification that the study participant signed the informed consent document by including one of the following:
- First page and signed Signature Page of the consent form
- Documentation on physician’s order sheet of date and time that the informed consent was signed
The drug will be prepared and dispensed per protocol specifications and established pharmacy policies. Dispensed study medication will be labeled with the Pharmacy Department’s computer-generated label, which conforms to state and federal law. The sponsor’s required labeling will be attached to the dispensed product in addition to the pharmacy label. No parts of the sponsor’s label will be obliterated by the pharmacy label. All study medications will be labeled with the caution, “For Investigational Use Only.” The administration of investigational drugs while in an Ambulatory Care Center, Infusion Room, or while admitted to the hospital, is the responsibility of the principal and co-investigators identified in the study protocol. An investigational medication may only be administered according to protocol and institutional specifications.
The IDS Pharmacy staff will meet with study monitors/auditors in order to assure protocol compliance/adherence. The study coordinator or study monitor must schedule the monitoring visit with the IDS Pharmacy at least two weeks in advance. The IDS Pharmacy will schedule a maximum of three monitor visits or a total of five “monitoring hours” per day.
The investigator must provide the pharmacy with written permission to unblind a subject’s treatment. This may be in the form of a written order or an email. The IDS pharmacy will unblind the patient and place a copy of the written correspondence in the study file/binder.
IDS Pharmacy Policies may be viewed on the Infonet (internal access only; login required). The list below includes some of the available IDS policies:
- Ordering and Dispensing Investigational Drugs
- Temperature Monitoring of Investigational Drugs in the Pharmacy
- Essential Document Handling and Retention
- Cost Estimate
- Destruction of Investigational Drugs
- Dispensing Investigational Drugs to a Home Health Care Agency
- Drug Accountability, Inventory Management and Returns
- Handling Investigational Biosafety Level 2 Products BSL2
- Monitors and Auditors
- NCI-Registered Investigators to Prescribe CTEP-Supplied Agents
- Pharmacy Staff Training for Investigational Drug Studies
- Storage of Investigational Biosafety Level 2 BSL2
- Transport of Investigational Drugs by Penn State Health Milton S. Hershey Medical Center Pharmacy
- Use of Investigational Drugs
Clinical Trial Maintenance
It is advisable that you consult the Penn State University IRB Investigator Manual (HRP-103) in the CATS IRB Library for details regarding changes to the study team or Other parts of the study and protocol exceptions prior to preparing your application. You must report planned changes in a study and receive approval from the IRB prior to implementing these changes, except where necessary to eliminate apparent immediate hazards to the subjects. Refer to the Expanded Access Submissions page on the HRPP website for instructions. In the case of changes implemented to eliminate immediate hazards to the subjects, the emergency protocol changes must be reported to the IRB using a Reportable New Information submission within 5 business days. See New Information that Needs to be Reported to the IRB During the Course of the Study.
To request modifications to an approved study, click “Create Modification / CR” in the CATS IRB system, answer the questions on each screen, attach all requested supporting documents and click the “Submit” activity in the workspace to send it to the IRB Office for review. When revising previously approved documents, such as protocols, consent forms, recruitment materials, etc., use a tracked changes feature and a version date to denote all revisions. Maintain electronic copies of all documents submitted to the IRB in case revisions are required. Please note that research must continue to be conducted without inclusion of the modification until IRB approval is received.
A protocol exception is a one-time, intentional action or process that departs from the IRB-approved study protocol, intended for one occurrence or applied to a single individual. This action must be approved prior to its implementation by the following:
- the sponsor or funding agency
- the FDA (if applicable)
An example of an exception may include: the potential enrollment, following approval of the sponsor, of a subject who fails to meet all of the protocol eligibility criteria. To request a protocol exception for an approved study, click “Create Modification / CR” in the CATS IRB system. In the modification summary section, provide the following information about the protocol exception:
- Description of the protocol exception, including a reference (page number or section) in the IRB-approved protocol that is being altered
- Justification for the protocol exception
- Discussion of the impact on the risks and/or benefits
- Discussion of the impact on the overall safety of the subject
- Discussion of the impact on the overall validity of the study
- Indication if the exception will be discussed with the subject and the rationale for this decision
- Indication of when the approval of the protocol exception is needed
If applicable, attach the sponsor’s and/or FDA’s approval of the protocol exception on the Local Site Documents page under question 3 Other attachments in CATS IRB. Click the “Submit” activity in the workspace to send it to the IRB Office for review.
For any study that requires continuing review, a continuing review form must be submitted prior to the expiration date of IRB approval. Where continuing review is required, the approval letter will indicate this. The IRB sends out multiple courtesy notices starting at approximately 90 days prior to the approval expiration date. It is the Principal Investigator’s responsibility to ensure the required information is submitted by the administrative due date in order to receive renewed approval prior to the expiration date. If the continuing review application is not received at least 6 weeks prior to expiration, the IRB may not be able to conduct a timely review, which may result in a lapse of IRB approval. Repeated instances of late submissions that result in a lapse of approval may be considered serious and/or continuing non-compliance. It is advisable that you consult the Penn State University IRB Investigator Manual (HRP-103) in the CATS IRB Library prior to preparing the continuing review form. To submit a continuing review, click “Create Modification /CR/Admin Review” in the CATS IRB system, answer the questions on each screen, attach all required supporting documents and click the “Submit” activity in the workspace to send it to the IRB Office for review. Maintain electronic copies of all information submitted to the IRB in case revisions are required.
If the continuing review involves a minor modification to previously approved research (e.g., adding a study team member or correcting a typographical error on a consent document), choose ‘Modification and Continuing Review’ on the first screen and submit those modifications as part of the continuing review. IMPORTANT: If the requested changes are more than minor changes, you must complete and submit the ‘Continuing Review’ submission and a separate ‘Modification’ submission. Also note that combined Modification and Continuing Review submissions must be processed and reviewed together (i.e., a minor modification will be approved with the continuing review, not before). If you expect one submission to be reviewed and approved before the other, then submit separate submissions (one modification, and a separate continuing review).
If IRB approval lapses, all Human Research procedures related to the protocol under review must cease including recruitment, advertisement, screening, enrollment, consent, interventions, interactions, and collection or analysis of private identifiable information. Continuing Human Research procedures without IRB approval is a violation of institutional policy and in some cases a violation of federal regulations. If current subjects will be harmed by stopping Human Research procedures that are available outside the Human Research context, provide these on a clinical basis as needed to protect current subjects. If the PI believes that current subjects will be harmed by stopping Human Research procedures that are not available outside the Human Research context, immediately contact the HRPP at [email protected] and provide a written list of the currently enrolled subjects and why they will be harmed by stopping procedures. Remember that research data cannot be collected when a study has lapsed. In addition, the HRPP will administratively close any study that is in a lapsed state for more than 45 days. Once closed, these studies cannot be re-opened and a new submission would have to be completed to continue any human research activities.
Annual Notices
Certain studies approved on or after Jan. 21, 2019, do not require continuing review; however, investigators will receive an annual notice in the study submission as a reminder that the principal investigator remains responsible to comply with all Investigator Responsibilities outlined in this manual until the principal investigator requests that the study is closed through a continuing review (CR) submission.
Principal Investigators responsibilities include, but are not limited to:
- Submitting any modifications that require approval (See Section: Regulatory Classifications of Research (Not Human, Exempt, Expedited, Full/Convened IRB and Exempt Modifications ))
- Submitting any reports of new information to the IRB (See Section: Reportable New Information (RNI) –Information to be Reported to the IRB )
- Submitting a continuing review (CR) to close the study when the research is complete (See Section: Study Closure Information)
Reporting under IND (Protocol Amendments)
You need to submit an IND Protocol Amendment if you have either of the following changes during the course of your study:
- New protocol
- Change in protocol
- New investigator (new site)
The study may begin after you obtain IRB approval based on the new or amended protocol and after the FDA receives the amendment. FDA does not issue “permissions” or “approvals” for protocol amendments, your changes are effective immediately upon the receipt of your amendment by the FDA. The IRB may request documentation of FDA review of amendments and may hold approval until documentation is received from the FDA. In these cases, the PI must request that the FDA provide documentation that the research may continue. For changes in the protocol , the IND Protocol Amendment consists of:
- Cover Letter identifying the submission as “Protocol Amendment: Change in Protocol” or “Protocol Amendment: New Protocol”
- Form 1571 – Check an appropriate box under Paragraph 11, “Protocol Amendments”
- A document outlining the differences between the new protocol and the original protocol
For changes in the investigators , the IND Protocol Amendment consists of:
- Cover letter identifying the submission as “Protocol Amendment: New Investigator”
- Form 1572 for the new investigator
If there are manufacturing or other changes, such as:
- Changes in chemistry, manufacturing and control,
- Changes in pharmacology/toxicology (new findings affecting safety and efficacy), or
- Decision to discontinue a clinical study,
the manufacturer (in many cases, industry sponsor) will notify you. Your responsibility is to notify the IRB and make a decision as to whether to proceed with your trial.
Reporting under IDE (IDE Supplements)
Any changes in the Investigational Plan should be approved by the FDA and, when appropriate, IRB, prior to implementing any change to a previously accepted Investigational Plan. The following types of protocol changes would require an approved IDE Supplement, because they are likely to have a significant effect on the scientific soundness of the trial design and/ or validity of the data resulting from the trial.
- Change in indication
- Change in type or nature of study control
- Change in primary endpoint
- Change in method of statistical evaluation
- Early termination of the study (except for reasons related to patient safety)
- Change in the number of investigational sites
- Change in the number of study subjects
However, if the modifications meet certain criteria, the sponsor of an IDE may modify the device and/or clinical protocol without prior FDA approval. The sponsor still needs to provide notice to FDA within five working days of making the change. These notices must be identified as a “notice of IDE change.”
- Emergency use: If PI deviates from the investigational plan to protect the life or physical well-being of a subject in an emergency. Such deviations should be reported to the IRB promptly after its occurrence, and to the FDA within five working days after the sponsor becomes aware of it.
- Certain changes to the device: Advance IRB notification is not required if the changes do not constitute a significant change in design or basic operation and are made in response to information gathered during the course of an investigation. Examples include: creditable data generated under the device control procedures (21 CFR Sec. 820.30), preclinical/animal testing, peer reviewed published literature, and clinical information gathered during a clinical trial or marketing.
- Certain clinical protocol changes: When they do not affect (i) the validity of the data or information resulting from the completion of the approved protocol, or the relationship of the likely patient risk to benefit ratio relied upon to approve the protocol; (ii) the scientific soundness of the investigational plan; or (iii) the rights, safety, or welfare of human subjects involved in the investigation.
- If changes will be submitted in the annual report: A sponsor may make minor changes to an Investigational Plan without prior FDA approval; provided that the respective changes are reported in the annual progress report for the IDE (see Annual Reports).
For details, see the RQA section of the Infonet (internal access only; login required).
Adverse Event (AE): An adverse event is an undesirable and unintended event occurring as a result of therapy or other intervention (e.g., headache following spinal tap or intestinal bleeding associated with aspirin therapy). It also includes any untoward or unfavorable medical occurrence in a human subject, including any abnormal sign (for example, abnormal physical exam or laboratory finding), symptom, or disease, temporally associated with the subject’s participation in the research. Serious Adverse Event (SAE): Events are classified as serious if they meet any of the following criteria:
- Results in death or any life threatening event that places the subject at immediate risk of death from the event as it occurred.
- Any event that requires or prolongs in-patient hospitalization.
- Any event that results in persistent or significant disability/incapacity.
- Any congenital anomaly/birth defect diagnosed in a child of a subject who participated in the study and received study drug.
- Other medically important events that in the opinion of the investigator may jeopardize the subject or may require intervention to prevent one of the other outcomes listed in the definition above.
Unanticipated AE: Any adverse experience, the frequency or severity of which is not consistent with the current consent form or investigator brochure. Unanticipated Problem Involving Risk to Participants or Others: Any unanticipated event involving any aspect of a research study involving anyone (participants, research staff, or others not directly involved in the research) that increases the risk to the person involved. See DHHS Guidance on Reviewing and Reporting Unanticipated Problems Involving Risks to Subjects or Others and Adverse Events (includes flowcharts and diagrams)
Once an adverse event becomes serious, the site should inform the Sponsor by submitting an SAE report. Typically, the Sponsor will provide the report form to use and inform the study investigator/coordinator where and how (i.e. email, fax, etc.) to send the report. An SAE report should be submitted to the Sponsor no later than 24 hours after the site becomes aware of the event. As the site gains more information (i.e. admission records, hospital discharge summaries) updated SAE reports with the new information should be submitted to the Sponsor. In this case the Sponsor (Industry/cooperative group) holds the IND and is therefore responsible for deciding whether the SAE should be reported to the FDA.
IND Safety Reports
In cases where the PI is both the Investigator and the Sponsor, the PI assumes the responsibility of reporting certain SAEs to the FDA. Once it is determined that an SAE must go to the FDA an IND Safety Report is prepared (usually the PI, in association with the medical monitor, will determine whether an IND Safety Report needs to be prepared). An IND Safety Report is an expedited, written notification to the FDA of an adverse experience associated with the use of a study drug that is both serious and unexpected.
When to file:
- For any unexpected fatal or life threatening SAE associated with the use of the drug, the IND Sponsor-Investigator notifies the FDA of the SAE by telephone or fax as soon as possible, but no later than seven calendar days after initial receipt of the SAE. The investigator follows with the written report no later than 15 days after the occurrence.
- For serious and unexpected, but non-fatal adverse events, file as soon as possible and no later than 15 days after initial receipt of the SAE.
For more on filing requirements and follow-up, see IND Application Reporting: Safety Reports .
IDE Safety Reports
An unanticipated adverse device effect is any serious adverse effect on health or safety, any life-threatening problem or death caused by, or associated with a device, if that effect, problem, or death was not previously identified in nature, severity, or degree of incidence in the application; or any other unanticipated serious problem associated with a device that relates to the rights, safety, or welfare of subjects. An investigator shall submit to the sponsor and to the reviewing IRB a report of any unanticipated adverse device effect occurring during an investigation as soon as possible, but in no event later than 10 working days after the investigator first learns of the effect. If the Investigator is a Sponsor-Investigator, he/she will notify the FDA and all participating investigators in a written IDE safety report of any unanticipated adverse device effects. The report is also provided to the device manufacturer and to the reviewing IRB as soon as possible, but no later than 10 working days after the Investigator first learns of the effect. Thereafter the sponsor (or Sponsor-Investigator) shall submit such additional reports concerning the effect as FDA requests. See IDE report details on the FDA website .
In many cases Sponsors will specify at the beginning of the study how they would like to handle protocol deviations. Minor deviations (as described elsewhere in this Guidebook) are usually recorded in the case report forms and tabulated by site at the end of the study. Most Sponsors do not require that minor deviations be reported in any immediate fashion. For major deviations the site often reports to the Sponsor are required. In the case where a site needs a deviation in order to enroll a patient that is not otherwise eligible per the protocol inclusion/exclusion criteria, a Sponsor will request that a planned protocol deviation be filed requesting permission from the Sponsor for the site to enroll the patient. Sponsors will respond to this request in writing and this form along with documentation of all communication between the site and Sponsor should be kept in the patient’s source documentation. IRB approval is also needed for these one-time protocol exceptions.
Reporting Protocol Deviations under IND
(Information adapted from www.firstclinical.com ) FDA’s regulations have numerous references to “changes” or “amendments” to study protocols. For example, 21 CFR 312.30 addresses the responsibility of sponsors to submit amendments to their IND(s) to ensure that clinical investigations are conducted according to protocols included in the application. 21 CFR 312.30(b) specifically discusses changes in a protocol, and provides several examples of changes that would require sponsors to submit protocol amendments to the IND. However, the FDA regulations do not provide specific guidance on deviation reporting. A protocol deviation directed at eliminating an apparent immediate hazard to a research subject or group of subjects may be implemented immediately provided that the reviewing IRB is so notified. The respective protocol deviation should be addressed in the next Annual Report to the IND application. If the protocol deviation will be incorporated as a permanent change (i.e., revision) to the protocol, a respective Protocol Amendment must be submitted prospectively to the IND application/FDA and the revision to the protocol must be approved prospectively by the responsible IRB.
Reporting Protocol Deviations under IDE
FDA device regulations at 21 CFR 812.150(a)(4) discuss protocol deviations under IDE regulations. An investigator shall notify the sponsor and the reviewing IRB of any deviation from the investigational plan to protect the life or physical well-being of a subject in an emergency. Such notice shall be given as soon as possible, but in no event later than five working days after the emergency occurred. Except in such an emergency, prior approval by the sponsor is required for changes in or deviations from a plan, and if these changes or deviations may affect the scientific soundness of the plan or the rights, safety, or welfare of human subjects, FDA and IRB should be made aware in accordance with 812.35(a).
Annual Reports to CDER
For clinical trials being conducted under an IND, FDA requires an annual report from the Sponsor or Sponsor-Investigator. The annual report is due within 60 days of the anniversary date that the IND went effect (i.e., the date that the FDA permitted the study to begin). Required content is listed in 21 CFR 312.33. See the RQA Infonet section for details (internal access only; login required).
Annual Reports to CDRH
For clinical trials being conducted under an IDE, FDA requires Sponsors to submit progress reports, at regular intervals, and at least yearly. Reports must be submitted to all reviewing IRBs and in the case of significant risk devices the sponsor must also submit the progress report to FDA (21 CFR 812.150). See the RQA Infonet section for details (internal access only; login required).
“Essential documents are those documents which individually and collectively permit evaluation of the conduct of the trial and the quality of the data produced. These documents serve to demonstrate the compliance of the investigator, sponsor and monitor with the standards of Good Clinical Practice and with all applicable regulatory requirements.” (ICH Guideline E6) There are many ways to organize essential documents, and there is no gold standard for how to do this. For example, the ICH GCP E6 guideline recommends that the documents be grouped according to the stage of the trial, i.e. documents relevant to the trial before it commences, documents relevant to the trial during the conduct of the trial, and those documents relevant to the trial after completion or termination of the trial. See specific information here . The most important thing is that the documentation is organized and that all of the necessary documents are present. This section of the Guidebook provides examples of a potential system to organize essential documents. Essential documents also serve a number of other important purposes. Filing essential documents at the investigator/institution and sponsor sites in a timely manner can greatly assist in the successful management of a trial. These documents are also the ones which are usually audited by the independent audits and inspected by the regulatory authority(ies) as part of the process to confirm the validity of the trial conduct and the integrity of data collected. Another way to organize the essential documents into study binders is by the content of the binder. For example, many sites have a “source document binder,” a “case report form binder,” a “financial binder” and a “regulatory binder.”
Industry sponsors may provide investigators with a regulatory binder to be used to maintain the essential documents for the trial.
Investigators who are conducting investigator initiated trials are encouraged to use either of the two resources available to maintain essential documents.
These two resources are:
- The Virtual Regulatory Binder inserts for regulatory documents maintained in paper format.
- REDCap eRegulatory Binder for electronic storage and organization of regulatory documentation.
The binder tab inserts and instructions, as well as additional information regarding access and utilization of the REDCap eRegulatory Binder, can be found via the Clinical Trials Office .
The following list represents the required essential documents that must be filed in the regulatory binder. All essential documents must be available for audit/inspection by the sponsor and regulatory authorities.
The Virtual Regulatory Binder adapted from Partners Healthcare provides all essential tabs and information about what needs to go under each tab.
Delegation of Authority/Responsibilities Log
It is common practice for investigators to delegate certain study-related tasks to employees, colleagues, or other third parties (individuals or entities not under the direct supervision of the investigator).
However, the Principal Investigator (PI) is ultimately responsible for the conduct of the study.
When tasks are delegated by an investigator, the investigator is responsible for providing adequate supervision of those to whom tasks are delegated. A Delegation of Authority log should be created documenting delegated tasks to delegated individuals. The same applies to staff/contract organizations no in direct employ of the investigator.
Title of the study
Below the log, the PI should sign and date.
Training Log
The investigator has to assure that the staff has appropriate education, training and experience to perform delegated tasks.
The training log should also document that individuals have been trained on protocol-specific topics relevant to their job responsibilities. This training is documented in the training log.
The investigator should develop a plan for the supervision and oversight of the clinical trial at the site. Supervision and oversight should be provided even for individuals who are highly qualified and experienced.
Such a plan is outlined in the FDA Guidance on Investigator Responsibilities and may include routine meetings, procedures for reviewing staff performance, procedures for correction of protocol deviations, and procedures for ensuring quality control.
Per ICH GCP guideline E6 section 5.1 source data is identified as “all information in original records of clinical findings, observations, or other activities in a clinical trial necessary for the reconstruction and evaluation of the trial.” This is the first recording of subject-related information. According to 21 CFR 312.62(b), and investigator is required to prepare and maintain adequate and accurate case histories that record all observations and other data pertinent to the investigation on each individual. Source documents must be complete, accurate, and valid. The regulatory authorities consider source documents to be the basis for al trial data and the adjudication of the outcome of events. The purpose of source documents/patient record binder:
- To document the existence of the participant and substantiate integrity of trial data collected.
- To include original documents related to the trial, medical treatment, history of participant, and participant’s condition while on-study or in follow-up.
- To provide an auditable link in the chain from the study database back to the original source (visit worksheet).
- To collect data for transfer to CRFs and then to the study database.
- To instruct study coordinators and other site personnel on what data to collect and information necessary to answer data queries.
According to ICH GCP EC 1.11, a case report form is a printed, optical, or electronic document designed to record all of the protocol required information to be reported on each trial subject. CRFs are designed by the sponsor or sponsor-investigator and maintained at the investigative site. Information documented on the CRF (or eCRF) must be supported by source documentation. At a minimum the CRF should record:
- Inclusion/exclusion criteria and assessment as to whether the subject met them
- Protocol-specific clinical laboratory testing (including EKGs, X-rays, eye exams, scans, etc.) documented by laboratory records
- All AEs, SAEs, concomitant therapies, and/or inter-current illnesses
- Assessment of severity of AEs, relationship to test article, and expectedness of the AE
- Report of all dropouts and the reasons
One of the most essential tasks performed by the Clinical Research Coordinator (CRC) is completing and/or ensuring the completion of the subject’s CRF. Most sponsors will provide instructions or a guide for CRF completion. Handwriting must be legible and should be completed in black ink. All data points must be addressed and for fields that cannot be completed, “not available,” “not done” or “unknown” should be marked in accordance with the sponsor’s instructions. The CRC will ensure that all required data are collected and entered on the CRF as soon as possible after, if not during, the visit. All CRFs should be checked for completeness and legibility. The CRFs should be reviewed and signed by the investigator prior to submission. Only those physicians identified on the 1572 may sign CRFs. When making a correction on a CRF, a single line should be drawn through the incorrect entry and the correct data should be entered above or next to the incorrect entry. The correction should be dated and initialed. White-out or eraser should never be used to correct an error. Blanks identified prior to the investigator’s review and sign-off on the CRF can simply be completed. Those identified after sign-off must be dated and initialed.
An electronic copy of the financial documents is kept in the individual study network folder. Studies are organized by department then by Investigator. The Controller’s office maintains electronic copies of all documents which include check copies, invoices, budget documents, etc. The documents in the network folder are kept separately from the negotiation items.
The following is an outline of the documents that should be kept in the financial binder:
An audit is a systematic and independent examination of trial-related activities and documents to evaluate whether the trial-related activities were conducted and the data were recorded, analyzed and accurately reported according to the protocol, Sponsor’s SOP, GCP and other applicable regulatory requirements. Auditors collect evidence and compare against standards, review documents, assess deviations and non-compliance and recommend actions.
The Bioresearch Monitoring Unit of the FDA may conduct inspections of medical research and testing facilities in order to ensure studies avoid bias and follow proper testing procedures.
The FDA inspector will review all case study data and may interview subjects and doctors. In all types of inspections, an FDA inspector checks the study for errors that affect the outcome.
Investigators may expect the following types of inspections:
- Routine Inspection may be conducted at random. It is sometimes triggered by abnormally high enrollment rate as well as large studies to promote a pivotal drug.
- For-Cause Inspections: FDA investigator has a reason to check out a research facility, i.e., subject complaint, a highly publicized drug, unqualified investigators, large AE clustering.
Once you receive notification of the FDA audit notify the appropriate research administration offices and IDS. Specific procedures to follow when preparing for an inspection and on the day of the inspection will be discussed with the research team prior to the inspection date and are outlined in the Standard Operating Procedures for Clinical Research, QA 601.
Also, see FDA guidance on FDA Inspection of Clinical Investigators for details.
Office of Research Quality Assurance (RQA) fulfills the auditor role for investigator-initiated studies. These audits are called Post Approval Reviews (PAR).
RQA conducts for-cause/directed reviews (requested by the IRB), random/routine reviews and self-evaluation assessments. The purpose of routine/random reviews is to assist investigators with achieving high quality of regulatory compliance. The reviews are meant to be more educational rather than punitive in nature.
RQA summarizes and reports the findings directly to the investigators. Investigators are required to submit all directed PAR reports to the IRB and significant findings in other PAR reports according to IRB Reportable New Information (RNI) reporting policy.
See RQA details.
If you have concerns about your preparedness for an audit, please contact the RQA office. RQA offers audit readiness assessment for both industry and investigator-initiated studies. This program helps ensure compliance with FDA, GCP, and IRB regulations, and institutional SOPs and policies and procedure as related to clinical research. The results of the pre-audit assessment will be provided for investigators and teams. For further information, contact RQA.
Billing and Invoicing
On a monthly basis the expense and income should be verified for the clinical research account. Reports are available via SIMBA, ran by the College of Medicine Research Accountant Supervisor. The COM Research Accountant, Department Financial Analyst and members of the study team should be certain that effort is being applied appropriately and that all income due is being tracked. The income and expenses in the accounting system should match the participant tracking document and any reports generated by hospital finance.
Within two business days, the study team is to enter participant visit information into STAR . In the event a research visit will take multiple days to complete, the study team should create the visit in STAR and record the date of the activities as they occur. The study team should not mark the visit as “completed” until all applicable items/services have been done. Since Patient Financial Services (PFS) is primarily focused on completed visits, it is recommended that the study team send the following message to PFS through STAR (edit as needed):
For this study, the XXX visit(s) will take place over an extended period (possibly xx weeks). I will keep the visit(s) updated and open as a snapshot of what has been done and what hasn’t occurred yet. I will not finalize the visit until it has been completed. Please feel free to call if you have any questions.
The study team should be reviewing the contract notes and notifying the financial contact of any events that require an invoice or any other items of interest.
For legacy studies (studies not in STAR)
On a weekly basis, the study team should enter the necessary visit information into the participant tracking Excel spreadsheet (on the shared network drive) and send to PFS and the central Financial Analyst (FA).
In the log, the first three columns should be filled out as listed. The rest of the columns, except for “Notes,” should be used to list the appropriate dates for that study. Any screening fails should be listed in notes and the appropriate subsequent dates grayed out. For questions filling out this Excel sheet, contact the Clinical Trials Office . A central FA uses this information to convert completed visits into Receivables by using the contracted amounts per visit/procedure. All costs for rescheduled visits, delayed procedures, adverse events and any other “invoiceable” items are also captured at this time.
In the event a research visit will take multiple days to complete, the study team should send the above referenced message PFS by email to [email protected] , notifying them of the extended visit period.
The COM Research Accountant should invoice the sponsor on a monthly or quarterly basis dependent upon the contract terms. The study team should collaborate with the COM Research Accountant in order to make sure all appropriate items are being invoiced. Communicate with the COM Research Accountant for more details in regards to invoices.
PI effort will be reviewed at a minimum of quarterly and applied where appropriate, in a timely manner, in accordance with the internal budget and subject accrual or protocol activity.
The following is to be used to determine what document is be used as the internal budget:
( Funding source: Document)
Grant: Budget document submitted as part of the grant application Industry contract: Budget Worksheet created by Clinical Trials Office Departmental funds: Department-specified document
Effort will be charged based on prorated accrual, not based on income received. Adjustments of actual effort vs. budgeted effort may not be greater than a 25 percent change, unless there is a contractual amendment to the agreement. Salary Assignment Schedules will be reviewed at a minimum of quarterly and updated to reflect appropriate effort, where applicable. For additional details, refer to the following policies:
- Policy: RAG64, Salary Caps
- Policy: RA30, Facilities and Administrative (F&A) Costs
Study Closure
Account Closure or Extension Forms (ACE Forms) are sent to the COM Research Accountant when an account has an end date in the accounting system. The Controller’s office receives a restricted fund report from Research Accounting at University Park with accounts that have end dates. The COM Research Accountant works with the investigator and study team to either extend the end date if the contract allows, request an amendment from the sponsor or close out the account in the accounting system. Communicate with the COM Research Accountant in order to obtain more details about the closure or extension of an account.
To request study closure, click “Create Modification/CR” in the CATS IRB system, answer the questions on each screen, attach all requested supplements and submit it to the IRB Office. Maintain electronic copies of all information submitted to the IRB in case revisions are required. Reference the Investigators Manual (HRP-103) in the CATS IRB Library for further information.
At the end of a trial, a close-out visit must be arranged by the study monitor. The monitor will perform the final drug reconciliation. The perpetual inventory will be “zeroed out” and the drug will be disposed of/mailed back per protocol. Copies of drug accountability records will be provided to the study monitor, which may be done by allowing the monitor access to Vestigo. Original records will not be released to the study monitor unless written permission from the study sponsor is obtained/provided by the study monitor. Once a study has been officially closed to accrual and all subjects at our institution have completed therapy with the supplied medication, the sponsor must perform final drug reconciliation within 30 days. After 30 days, any remaining drug will be destroyed per policy PAM 1406 (Investigational Drug Services: Destruction of Investigational Drugs.) Refer to the IDS section of this guidebook for additional information.
Once the project is terminated by the sponsor or the contract end date expires, the Controller’s Office receives a monthly report from Research Accounting. The Controller’s Office notifies the appropriate financial administrator for closeout or extension and provides an Account Closeout/Extension (ACE) form for completion. Once the financial administrator returns the ACE form to the Controller’s Office, the Controller’s Office notifies ORA of the expiration. ORA updates the SIMS database. Contracts must retain the agreement for the period of time designated in the agreement or if not so designated the period legally required The PI and department must retain the project records for the period of time designated in the agreement.
For drugs, according to 21 CRF 312.62(c), an investigator shall retain records required to be maintained under the part for a period of two years following the date a marketing application is approved for the drug for the indication for which it is being investigated; or, if no application is to be filed or if the applications is not approved for such indication, until two years after the investigation is discontinued and FDA is notified.
For devices, according to 21 CRF 812.140(d), an investigator or sponsor shall maintain the records required by this subpart during the investigation and for a period of two years after the latter of the following two dates: the date on which the investigation is terminated or completed, or the date that the records are no longer required for purposes of supporting a premarket approval application or a notice of completion of a product development protocol.
Study Sponsors may have additional document retaining provisions stipulated in the Contract.
The National Institutes of Health (NIH) requires researchers to acknowledge federal funding in peer-reviewed publications by citing any NIH grants that supported the research process described in the publication. In addition, the NIH Public Access Policy requires that all investigators “funded by the NIH,” be it through direct funding or through use of resources of an NIH-funded center (such as Penn State Clinical and Translational Science Institute) submit an electronic version of their final, peer-reviewed manuscripts to PubMed Central (PMC) upon acceptance of publication. This policy ensures that the public has access to the published results of NIH-funded research. Failure to submit the manuscript to PMC within NIH-imposed deadlines may result in a delay of processing the grant awards of the researchers or centers whose grants were cited in the manuscript.
Resources about complying with the NIH Public Access Policy
- Details of the NIH PublicAccess Policy
- Directions and tutorials for submitting a manuscript to PMC through the NIH manuscript submission system
- Information about citing the CTSI in a publication
Research Support
- COVID-19 Research Guidance
- Research Support Overview
- Research and Innovation Awards
- Clinical Research Center
- Clinical Trials Office
- Study Tracking and Analysis for Research (STAR)
- Confidentiality and Non-Disclosure Agreements
- Conflict of Interest
- Institutional Animal Care and Use Committee (IACUC)
- Research Compliance Overview
- Commercialization and Innovation (Center for Medical Innovation)
- Corporate and Foundation Relations
- Research Affairs (ORA)
- Sponsored Research Highlights
- Animal Resources
- College of Medicine IT (Research Informatics)
- Core Facilities and Research Resources
- Penn State Research Portal (Pure Researcher Directory)
- Roles and Responsibilities Through the Research Lifecycle

Medical Research Guide
- Introduction
- Your Clinical Question
- Get Ready to Document Your Research
- Finding Resources for Your Research
- If your project includes human subjects
- Managing Your Project
- Writing Your Paper
- PowerPoint Presentation Guide
- Creating a Quality Poster Presentation
- "Where can I submit my research for publication consideration?"
Your Medical Librarian

Whether it is formal scientific research or personal patient-care oriented research, research is a way of life for the successful medical professional.
This guide introduces the "nuts and bolts" of the professional research process and to help guide you through your research project from its conception to its completion.
Campbell University's medical librarian is happy to assist you with selecting and narrowing a topic, formulating a clinical question, selecting databases, constructing and executing a literature search, and locating articles. You can contact the medical library at either 910-893-7700 during business hours, or [email protected] . If you prefer, you can contact one of the medical librarians directly at either [email protected] (Steve Bahnaman) or [email protected] (Sarah Wade).
Important: As you progress through this guide, remember to scroll down to the bottom of each page.
- Next: Your Clinical Question >>
- Last Updated: Nov 21, 2023 3:17 PM
- URL: https://guides.lib.campbell.edu/medicalresearch
- Encyclopedias & Subject Guides

Enjoy fast, free delivery, exclusive deals, and award-winning movies & TV shows with Prime Try Prime and start saving today with fast, free delivery
Amazon Prime includes:
Fast, FREE Delivery is available to Prime members. To join, select "Try Amazon Prime and start saving today with Fast, FREE Delivery" below the Add to Cart button.
- Cardmembers earn 5% Back at Amazon.com with a Prime Credit Card.
- Unlimited Free Two-Day Delivery
- Streaming of thousands of movies and TV shows with limited ads on Prime Video.
- A Kindle book to borrow for free each month - with no due dates
- Listen to over 2 million songs and hundreds of playlists
- Unlimited photo storage with anywhere access
Important: Your credit card will NOT be charged when you start your free trial or if you cancel during the trial period. If you're happy with Amazon Prime, do nothing. At the end of the free trial, your membership will automatically upgrade to a monthly membership.

Return this item for free
We offer easy, convenient returns with at least one free return option: no shipping charges. All returns must comply with our returns policy.
- Go to your orders and start the return
- Select your preferred free shipping option
- Drop off and leave!
| Returnable | Yes |
|---|---|
| Resolutions | Eligible for refund or replacement |
| Return Window | 30 days from delivery |
| Refund Timelines | Typically, an advance refund will be issued within 24 hours of a drop-off or pick-up. For returns that require physical verification, refund issuance may take up to 30 days after drop-off or pick up. Where an advance refund is issued, we will re-charge your payment method if we do not receive the correct item in original condition. See details . |
| Late fee | A late fee of 20% of the item price will apply if you complete the drop off or pick up after the ‘Return By Date’. |
| Restocking fee | A restocking fee may apply if the item is not returned in original condition and original packaging, or is damaged or missing parts for reasons not due to Amazon or seller error. See details . |

Return instructions
| Item must be in original condition and packaging along with tag, accessories, manuals, and inserts. Unlock any electronic device, delete your account and remove all personal information. |
Sorry, there was a problem.
0.69 mi | SANTA CLARASanta Clara 95050

Download the free Kindle app and start reading Kindle books instantly on your smartphone, tablet, or computer - no Kindle device required .
Read instantly on your browser with Kindle for Web.
Using your mobile phone camera - scan the code below and download the Kindle app.

Image Unavailable

- To view this video download Flash Player

Follow the author

The Comprehensive Guide To Clinical Research: A Practical Handbook For Gaining Insight Into The Clinical Research Industry
Purchase options and add-ons.
- ISBN-10 1090349521
- ISBN-13 978-1090349521
- Publication date April 21, 2019
- Language English
- Dimensions 6 x 0.55 x 9 inches
- Print length 219 pages
- See all details
Frequently bought together

Customers who bought this item also bought

Product details
- Publisher : Independently published (April 21, 2019)
- Language : English
- Paperback : 219 pages
- ISBN-10 : 1090349521
- ISBN-13 : 978-1090349521
- Item Weight : 11.5 ounces
- Dimensions : 6 x 0.55 x 9 inches
- #25 in Medical Research (Books)
- #52 in Medical Encyclopedias
- #191 in Medical Reference (Books)
About the author
My name is Dan Sfera and I am an entrepreneur currently involved in the clinical research industry. My first full length book is called "The Comprehensive Guide To Clinical Research". Throughout my career I have worked at the site, CRO, Sponsor and vendor levels of clinical research in some capacity. As a former study coordinator and current contract CRA and Site Owner, I am uniquely positioned to have a holistic perspective when it comes to the various intricacies of this industry. I enjoy writing about the clinical research industry as well as business in general.
Customer reviews
- 5 star 4 star 3 star 2 star 1 star 5 star 76% 16% 5% 0% 3% 76%
- 5 star 4 star 3 star 2 star 1 star 4 star 76% 16% 5% 0% 3% 16%
- 5 star 4 star 3 star 2 star 1 star 3 star 76% 16% 5% 0% 3% 5%
- 5 star 4 star 3 star 2 star 1 star 2 star 76% 16% 5% 0% 3% 0%
- 5 star 4 star 3 star 2 star 1 star 1 star 76% 16% 5% 0% 3% 3%
Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them.
To calculate the overall star rating and percentage breakdown by star, we don’t use a simple average. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon. It also analyzed reviews to verify trustworthiness.
Customers say
Customers find the book easy to read and a good general overview of the business. They also say it hits every aspect.
AI-generated from the text of customer reviews
Customers find the book's content good, general, and understandable. They say it provides a good overview of the industry and contains a lot of industry information. Customers also say the book is laid out well and hits on important topics relevant in clinical research.
" A good read . Good English. The font is good." Read more
"...definitely recommend this book because it was a thorough, yet concise overview of how clinical trial research is done at the site level, what the..." Read more
"This book is a comprehensive guide indeed . Look up the author on YouTube. The info he offers for free is the real deal...." Read more
"...The book is laid out well starting with research history and then going through trial start up through close out...." Read more
Customers find the book easy to read, with good English and a good font. They also say it's exceptionally written for the CRC.
"A good read. Good English. The font is good ." Read more
"...My son is going into this business and this is an easy read , it hits every aspect to help you understand the conduct of a clinical trial, it helps..." Read more
"Awesome! informational! Exceptionally written for the CRC " Read more
Reviews with images

- Sort reviews by Top reviews Most recent Top reviews
Top reviews from the United States
There was a problem filtering reviews right now. please try again later..
Top reviews from other countries
- Amazon Newsletter
- About Amazon
- Accessibility
- Sustainability
- Press Center
- Investor Relations
- Amazon Devices
- Amazon Science
- Sell on Amazon
- Sell apps on Amazon
- Supply to Amazon
- Protect & Build Your Brand
- Become an Affiliate
- Become a Delivery Driver
- Start a Package Delivery Business
- Advertise Your Products
- Self-Publish with Us
- Become an Amazon Hub Partner
- › See More Ways to Make Money
- Amazon Visa
- Amazon Store Card
- Amazon Secured Card
- Amazon Business Card
- Shop with Points
- Credit Card Marketplace
- Reload Your Balance
- Amazon Currency Converter
- Your Account
- Your Orders
- Shipping Rates & Policies
- Amazon Prime
- Returns & Replacements
- Manage Your Content and Devices
- Recalls and Product Safety Alerts
- Conditions of Use
- Privacy Notice
- Consumer Health Data Privacy Disclosure
- Your Ads Privacy Choices
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Medical research articles from across Nature Portfolio
Medical research involves research in a wide range of fields, such as biology, chemistry, pharmacology and toxicology with the goal of developing new medicines or medical procedures or improving the application of those already available. It can be viewed as encompassing preclinical research (for example, in cellular systems and animal models) and clinical research (for example, clinical trials).

Multiorgan biological age shows that no organ system is an island
In our study, we linked machine-learning-derived biological age gaps (BAGs) to common genetic variants in nine human organ systems, which revealed how these BAGs are causally associated with organ health and chronic diseases such as Alzheimer’s disease and diabetes. The findings provide insights into therapeutic and lifestyle interventions that might enhance organ health.
Integrating traditional and modern medicines to treat heart failure
A large clinical trial supports the incorporation of qiliqiangxin, a traditional Chinese medicine, into standard treatment regimens for heart failure — highlighting a promising integration of ancient wisdom with modern medical practices through rigorous science.
- Zhuang Tian
- Shuyang Zhang
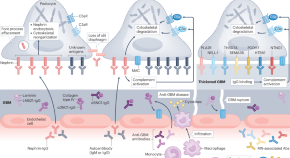
Anti-nephrin autoantibodies: a paradigm shift in podocytopathies
A new study demonstrates that anti-nephrin autoantibodies are not merely markers but also actively contribute to the pathogenesis of minimal change disease and primary focal segmental glomerulosclerosis. This insight not only provides a non-invasive diagnostic alternative to kidney biopsies, but also suggests potential for novel targeted therapies.
- Ming-hui Zhao
Related Subjects
- Drug development
- Epidemiology
- Experimental models of disease
- Genetics research
- Outcomes research
- Paediatric research
- Preclinical research
- Stem-cell research
- Clinical trial design
- Translational research
Latest Research and Reviews
Restoring initial steps by intermittent theta burst stimulation in complete spinal cord injury patient: a case report
- Deeksha Patel
- Rohit Banerjee

Causal role of immune cells in aplastic anemia: Mendelian randomization (MR) study
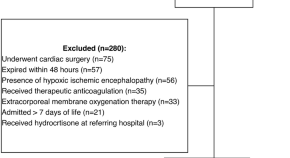
Incidence of gastrointestinal bleeding with hydrocortisone use in neonates and infants less than three months of age in the neonatal intensive care unit
- Dominique L. Ropp
- Peter N. Johnson
- Jamie L. Miller
Representational similarity analysis of neural patterns in childhood maltreatment
- Marika G. Del Motte
- Amanda J. F. Tamman

Correlation of spinal cord compression angle and increased signal intensity on MRI in patients with ossification of posterior longitudinal ligament
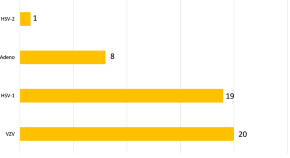
Utilization of multiplex polymerase chain reaction for simultaneous and rapid detection of viral infections from different ocular structures
- Arash Letafati
- Seyed Mohammad Jazayeri
- Azam Ghaziasadi
News and Comment
Food is brain medicine — relevance and translation to neurology.
The importance of diet for brain health is increasingly recognized by neurologists, but many neurological disorders impair the ability of individuals to eat healthily. A new initiative known as ‘Food Is Medicine’ has the potential to facilitate healthier eating among people with neurological disorders to improve and maintain brain health.
- Mitchell S. V. Elkind
- Kevin G. Volpp
Comment on: “A nationwide study of breast reconstruction after mastectomy in patients with breast cancer receiving postmastectomy radiotherapy: comparison of complications according to radiotherapy fractionation and reconstruction procedures”
- Jilong Yuan
Reply to RE: Can ChatGPT provide high-quality patient information on male lower urinary tract symptoms suggestive of benign prostate enlargement?
- Angie K. Puerto Nino
- Valentina Garcia Perez
- Dean S. Elterman
How psilocybin affects the brain
A longitudinal imaging study finds that psilocybin ‘desynchronizes’ the human brain, dissolving network connections linked to our sense of space, time and self — which could explain the therapeutic effects of this psychedelic.
- Karen O’Leary
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
Clinical Practice Guidelines
“Clinical practice guidelines are systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.” (Institute of Medicine, 1990)
Issued by third-party organizations, and not NCCIH, these guidelines define the role of specific diagnostic and treatment modalities in the diagnosis and management of patients. The statements contain recommendations that are based on evidence from a rigorous systematic review and synthesis of the published medical literature.
These guidelines are not fixed protocols that must be followed, but are intended for health care professionals and providers to consider. While they identify and describe generally recommended courses of intervention, they are not presented as a substitute for the advice of a physician or other knowledgeable health care professional or provider.
Featured Guideline
- National Guideline Clearinghouse (AHRQ)
- Institute of Medicine's Report Clinical Practice Guidelines We Can Trust (IOM)
- Travelers’ Health: Clinician Resources (CDC)
Allergy and Immunology
- Guidelines for the Diagnosis and Management of Asthma (NHLBI)
- Diagnosis and Management of Food Allergy (Journal of Allergy and Clinical Immunology) [ 165KB PDF]
- Allergic Rhinitis and Its Impact on Asthma (ARIA) Guidelines: 2010 Revision (Journal of Allergy and Clinical Immunology)
- Clinical Practice Guideline: Allergic Rhinitis (American Academy of Otolaryngology—Head and Neck Surgery) [ 1KB PDF]
- Vitamin, Mineral, and Multivitamin Supplements for the Primary Prevention of Cardiovascular Disease and Cancer (USPSTF)
- Soy Protein, Isoflavones, and Cardiovascular Health (Circulation)
- Management of Stable Ischemic Heart Disease (Annals of Internal Medicine)
Family Medicine
- Vitamin D and Calcium Supplementation to Prevent Fractures in Adults (U.S. Preventive Services Task Force)
- Treating Tobacco Use and Dependence: 2008 Update (AHRQ)
- Recommendations on Immunization (CDC)
- Practice Parameters for the Psychological and Behavioral Treatment of Insomnia: An Update (Sleep) [ 279 KB PDF]
- Practice Parameters for the Nonpharmacologic Treatment of Chronic Insomnia (Sleep)
- NSAIDs and Other Complementary Treatments for Episodic Migraine Prevention in Adults (Neurology) [ 393KB PDF]
- Guidance for the Prevention and Control of Influenza in the Peri- and Postpartum Settings (CDC)
- Guidance for Clinicians on the Use of Rapid Influenza Diagnostic Tests (CDC)
- Evaluation and Management of Chronic Insomnia in Adults (Journal of Clinical Sleep Medicine) [ 863 KB PDF]
- American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention (CA: A Cancer Journal for Clinicians) [ 450 KB PDF]
- 2011–2012 Influenza Antiviral Medications: Summary for Clinicians (CDC)
Gastroenterology
- ACG Clinical Guideline: Management of Irritable Bowel Syndrome (American Journal of Gastroenterology)
- Probiotics and Children (Journal of Pediatric Gastroenterology and Nutrition) [ 119KB PDF]
- Evidence-Based Recommendations on Management of Irritable Bowel Syndrome (American Family Physician)
Men's Health
- Management of Benign Prostatic Hyperplasia (BPH) (American Urological Association)
- Practice Parameter: Neuroprotective Strategies and Alternative Therapies for Parkinson Disease (An Evidence-Based Review) (Neurology)
- Complementary and Alternative Medicine in Multiple Sclerosis (American Academy of Neurology)
- Clinical Practice Guidelines on the Evidence-Based Use of Integrative Therapies During and After Breast Cancer Treatment (CA: A Cancer Journal for Clinicians)
- Clinical Practice Guidelines on the Use of Integrative Therapies as Supportive Care in Patients Treated for Breast Cancer (Journal of the National Cancer Institute Monographs)
- Exercise Guidelines for Cancer Survivors (Medicine & Science in Sports & Exercise)
Orthopedics
- Use of Supplemental Vitamin D and Calcium in Patients Following Hip Fracture Surgery (AAOS)
- Adjuvant Treatment of Distal Radius Fractures With Vitamin C (AAOS)
Pain Management
- Treatment of Osteoarthritis of the Knee (American Academy of Orthopaedic Surgeons)
- Subluxation Chiropractic Practice (Council on Chiropractic Practice)
- Practice Parameter: Evidence-based Guidelines for Migraine Headache (Neurology)
- Osteopathic Manipulative Treatment for Low-Back Pain (Journal of the American Osteopathic Association)
- Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline (Annals of Internal Medicine)
- Management of the Acute Migraine Headache (American Family Physician)
- Low Back Pain: Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability, and Health (Journal of Orthopaedic and Sports Physical Therapy)
- Diagnosis and Treatment of Low Back Pain (Annals of Internal Medicine)
- Practice Guidelines for Chronic Pain Management (Anesthesiology) [ 699 KB PDF]
- Chiropractic Management of Fibromyalgia Syndrome: Summary of Clinical Practice Recommendations (Council on Chiropractic Guidelines and Practice Parameters) [ 21 KB PDF]
- Management of Children with Autism Spectrum Disorders (Pediatrics)
- Pediatric Integrative Medicine (American Academy of Pediatrics)
- Migraine Headaches in Children and Adolescents (Journal of Pediatric Health Care)
- A Practice Pathway for Identification, Evaluation, and Management of Insomnia in Children and Adolescents with Autism Spectrum Disorders (Pediatrics)
Psychiatry and Mental Health
- Nonpharmacologic Versus Pharmacologic Treatment of Adults With Major Depressive Disorder (American College of Physicians)
- Clinical Guideline for the Evaluation and Management of Chronic Insomnia in Adults (PubMed)
Rheumatology
- Guidelines for the Non-Surgical Management of Knee Osteoarthritis (OA Research Society International) [ 5.2 MB PDF]
- Nonpharmacologic and Pharmacologic Therapies for Osteoarthritis of the Hand, Hip, and Knee (American College of Rheumatology/Arthritis Foundation)
Women's Health
- Treatment of Urinary Tract Infections in Nonpregnant Women (ACOG)
- Use of Botanicals for Management of Menopausal Symptoms (Obstetrics and Gynecology)
- AACE Medical Guidelines for Clinical Practice for Diagnosis and Treatment of Menopause © 2011 (Endocrine Practice)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.6(2); Winter 2006
Clinicians' Guide to Statistics for Medical Practice and Research: Part I
Marie a. krousel-wood.
* Ochsner Clinic Foundation, New Orleans, Louisiana
† Department of Epidemiology, Tulane School of Public Health and Tropical Medicine, New Orleans, Louisiana
Richard B. Chambers
Paul muntner, introduction.
This two-part series will present basic statistical principles for the practicing physician to use in his or her review of the literature and to the physician engaged in clinical research. The purpose of this series is threefold: (1) to provide an overview of common epidemiological and statistical terms and concepts that can be useful to the practitioner and clinical researcher, (2) to review calculations for common epidemiological measures and statistical tests, and (3) to provide examples from the published literature of uses of statistics in medical care and research. This review is not intended to be a comprehensive presentation of epidemiology or statistics since there are already a number of excellent sources for this information 1–6), but rather as a quick reference for practical application of statistical principles and concepts in medical care and clinical research.
In this issue, Part I of the Series is presented and includes discussion of the study question, study goals, appropriate study design, and appropriate statistical tests.
Physicians can be overwhelmed when reviewing published and current studies to determine what is relevant to their clinical practice and/or clinical research. Some initial questions outlined below may guide the process for reviewing an article or setting up a clinical study.
- What is the study question? What are the study goals?
- What is the appropriate study design to answer the study question?
- What are the appropriate statistical tests to utilize?
What Is the Study Question? What Are the Study Goals?
Whether in clinical practice or in a clinical research “laboratory,” physicians often make observations that lead to questions about a particular exposure and a specific disease. For example, one might observe in clinical practice that several patients taking a certain antihypertensive therapy develop pulmonary symptoms within 2 weeks of taking the drug. The physician might question if the antihypertensive therapy is associated with these symptoms. A cardiologist may observe in a review of the medical literature that the initial costs of caring for patients with cardiovascular diseases have been reported to be greater if the patient is cared for by a specialist than if the patient is cared for by a non-specialist. Because the physician may believe that although initial costs are greater, the follow-up costs are less, he or she may question if there would be a difference by specialist versus non-specialist if all costs were assessed. Questions like these can lead to formal hypotheses that can then be tested with appropriate research study designs and analytic methods. Identifying the study question or hypothesis is a critical first step in planning a study or reviewing the medical literature. It is also important to understand up front what the related study goals are. Some questions that may facilitate the process of identifying the study goals follow:
- Is the goal to determine:
- – how well a drug or device works under ideal conditions (i.e., efficacy)?
- – how well a drug or device works in a free-living population (i.e., effectiveness)?
- – the causes or risk factors for a disease?
- – the burden of a disease in the community?
- Is the study goal to provide information for a quality management activity?
- Will the study explore cost-effectiveness of a particular treatment or diagnostic tool? The hypotheses and the goals of a study are the keys to determining the study design and statistical tests that are most appropriate to use.
What Is the Appropriate Study Design To Answer the Study Question?
Once the study question(s) and goals have been identified, it is important to select the appropriate study design. Although the key classification scheme utilizes descriptive and analytic terminology, other terminology is also in vogue in evaluating health services and will be briefly described at the end of this section.
Classification Schemes
Epidemiology has been defined as “the study of the distribution and determinants of disease frequency” in human populations (4) . The primary classification scheme of epidemiological studies distinguishes between descriptive and analytic studies. Descriptive epidemiology focuses on the distribution of disease by populations, by geographic locations, and by frequency over time. Analytic epidemiology is concerned with the determinants, or etiology, of disease and tests the hypotheses generated from descriptive studies. Table 1 lists the study design strategies for descriptive and analytic studies. Below is a brief description of the various design strategies. The strengths and limitations of these study designs are compared in Table 2 .
Table 1: Outline of Study Design Strategies for Descriptive and Analytic Studies.

Table 2: Strengths and Limitations of Descriptive and Analytic Study Designs*

Descriptive Studies:
Correlational studies, also called ecologic studies, employ measures that represent characteristics of entire populations to describe a given disease in relation to some variable of interest (e.g. medication use, age, healthcare utilization). A correlation coefficient (i.e. Pearson's “r”; Spearman's “T”; or Kendall's “K”) quantifies the extent to which there is a linear relationship between the exposure of interest or “predictor” and the disease or “outcome” being studied. The value of the coefficient ranges between positive 1 and negative 1. Positive 1 reflects a perfect correlation where as the predictor increases, the outcome (or risk of outcome) increases. Negative 1 reflects a perfect inverse correlation where as the predictor increases the outcome (or risk of outcome) decreases. An example of a correlation study would be that of St Leger and colleagues who studied the relationship between mean wine consumption and ischemic heart disease mortality (7) . Across 18 developed countries, a strong inverse relationship was present. Specifically, countries with higher wine consumption had lower rates of ischemic heart disease and countries with lower wine consumption had higher rates of ischemic heart disease. Although correlation studies provide an indication of a relationship between an exposure and an outcome, this study design does not tell us whether people who consume high quantities of wine are protected from heart disease. Thus, inferences from correlation studies are limited.
Case reports and case series are commonly published and describe the experience of a unique patient or series of patients with similar diagnoses. A key limitation of the case report and case series study design is the lack of a comparison group. Nonetheless, these study designs are often useful in the recognition of new diseases and formulation of hypotheses concerning possible risk factors. In a case series study reported by Kwon and colleagues (8) , 47 patients were examined who developed new or worsening heart failure during treatment with tumor necrosis factor (TNF) antagonist therapy for inflammatory bowel disease or rheumatoid arthritis. After TNF antagonist therapy, 38 patients (of which 50% had no identifiable risk factors) developed new-onset heart failure and 9 experienced heart failure exacerbation. From this descriptive study, the authors concluded that TNF antagonist might induce new-onset heart failure or exacerbate existing disease (8) .
Cross-sectional surveys are also known as prevalence surveys. In this type of study, both exposure and disease status are assessed at the same time among persons in a well-defined population. These types of studies have become more common recently with the development and validation of survey tools such as the Short Form 36 (SF 36) functional status questionnaire and the Kansas City Cardiomyopathy Questionnaire (KCCQ) functional status survey. Cross-sectional studies are especially useful for estimating the population burden of disease. The prevalence of many chronic diseases in the United States is calculated using the National Health and Nutrition Examination Survey, an interview and physical examination study including thousands of non-institutionalized citizens of the United States. For example, Ford and colleagues estimated that 47 million Americans have the metabolic syndrome using the Third National Health and Nutrition Examination Survey (9) . Of note, in special circumstances where one can easily deduce an exposure variable preceding the outcome or disease, cross sectional surveys can be used to test epidemiologic hypotheses and thus can be used as an analytic study. For example, Bazzano and colleagues used data collected from a cross-sectional study to conclude that cigarette smoking may raise levels of serum C-reactive protein (10) . A cross-sectional study is useful in this situation because it is unlikely that having high levels of C-reactive protein would cause one to smoke cigarettes.
Analytic Studies:
Analytic studies can be observational or experimental. In observational studies, the researchers record participants' exposures (e.g., smoking status, cholesterol level) and outcomes (e.g., having a myocardial infarction). In contrast, an experimental study involves assigning one group of patients to one treatment and another group of patients to a different or no treatment. There are two fundamental types of observational studies: case control and cohort. A case control study is one in which participants are chosen based on whether they do (cases) or do not (controls) have the disease of interest. Ideally, cases should be representative of all persons developing the disease and controls representative of all persons without the disease. The cases and controls are then compared as to whether or not they have the exposure of interest. The difference in the prevalence of exposure between the disease/no disease groups can be tested. In these types of studies, the odds ratio is the appropriate statistical measure that reflects the differences in exposure between the groups.
The defining characteristic of a cohort study, also known as a follow-up study, is the observation of a group of participants over a period of time during which outcomes (e.g., disease or death) develop. Participants must be free from the disease of interest at the initiation of the study. Subsequently, eligible participants are followed over a period of time to assess the occurrence of the disease or outcome. These studies may be classified as non-concurrent/retrospective or concurrent/prospective.
Retrospective cohort studies refer to those in which all pertinent events (both exposure and disease) have already occurred at the time the study has begun. The study investigators rely on previously collected data on exposure and disease. An example of a non-concurrent/retrospective cohort study would be that of Vupputuri and colleagues (11) , who in 1999–2000 abstracted data on blood pressure and renal function from charts for all patients seen at the Veterans Administration Medical Center of New Orleans Hypertension Clinic from 1976 through 1999. They analyzed the data to see if blood pressure at each patient's first hypertension clinic encounter was associated with a subsequent deterioration in renal function.
In prospective studies, the disease/outcome has not yet occurred. The study investigator must follow participants into the future to assess any difference in the incidence of the disease/outcome between the types of exposure. The incidence of the disease/outcome is compared between the exposed and unexposed groups using a relative risk (RR) calculation. The advantages of retrospective cohort studies, relative to prospective, include reduced cost and time expenditures as all outcomes have already occurred. In contrast, the major disadvantage of the non-concurrent/retrospective studies is the reliance on available data that were collected for clinical purposes and, generally, not following a carefully designed protocol. There are two additional sub-classifications for cohort studies. First, cohort studies may include a random sample of the general population, e.g., Framingham and Atherosclerosis Risk in Communities (12–16) or a random sample of a high-risk population (17) . In these latter studies, a sample of all individuals or individuals with a specific demographic, geographic, or clinical characteristic is included. Second, cohort studies may begin by identifying a group of persons with an exposure and a comparison group without the exposure. This type of cohort study is usually performed in the situation of a rare exposure.
Experimental or intervention studies are commonly referred to as clinical trials. In these studies, participants are randomly assigned to an exposure (such as a drug, device, or procedure). “The primary advantage of this feature (ed: randomized controlled trials) is that if the treatments are allocated at random in a sample of sufficiently large size, intervention studies have the potential to provide a degree of assurance about the validity of a result that is simply not possible with any observational design option” (5) . Experimental studies are generally considered either therapeutic or preventive. Therapeutic trials target patients with a particular disease to determine the ability of a treatment to reduce symptoms, prevent recurrence or decrease risk of death from the disorder. Prevention trials involve the assessment of particular therapies on reducing the development of disease in participants without the disease at the time of enrollment. One such prevention trial is the Drugs and Evidence Based Medicine in the Elderly (DEBATE) Study, which has as its primary aim “ to assess the effect of multi-factorial prevention on composite major cardiovascular events in elderly patients with atherosclerotic diseases” (18) .
Other Classification Schemes:
Some other classification schemes in use today are based on the use of epidemiology to evaluate health services. Epidemiological and statistical principles and methodologies are used to assess health care outcomes and services and provide the foundation for evidence-based medicine. There are different ways to classify studies that evaluate health care services. One such scheme distinguishes between process and outcomes studies. Process studies assess whether what is done in the medical care encounters constitutes quality care (e.g. number and type of laboratory tests ordered, number and type of medications prescribed, frequency of blood pressure measurement). An example of a process study would be one that evaluated the percentage of patients with chronic heart failure in a given population who have filled prescriptions for angiotensin converting enzyme inhibitors (ACE – Inhibitors). A criticism of process studies is that although they document whether or not appropriate processes were done, they don't indicate if the patient actually benefited or had a positive outcome as a result of the medical processes.
Outcomes studies assess the actual effect on the patient (e.g. morbidity, mortality, functional ability, satisfaction, return to work or school) over time, as a result of their encounter(s) with health care processes and systems. An example of this type of study would be one that assessed the percentage of patients with a myocardial infarction (MI) who were placed on a beta blocker medication and subsequently had another MI. For some diseases, there may be a significant time lag between the process event and the outcome of interest. This often results in some patients being lost to follow-up, which may lead to erroneous conclusions unless methods that “censor” or otherwise adjust for missing time-dependent covariates are used.
In reviewing the medical literature, one often encounters other terms that deal with the evaluation of medical services: efficacy, effectiveness, or efficiency. Efficacy evaluates how well a test, medication, program or procedure works in an experimental or “ideal” situation. Efficacy is determined with randomized controlled clinical trials where the eligible study participants are randomly assigned to a treatment or non-treatment, or treatment 1 versus treatment 2, group. Effectiveness assesses how well a test, medication, program or procedure works under usual circumstances. In other words, effectiveness determines to what extent a specific healthcare intervention does what it is intended to do when applied to the general population. For example, although certain anti-retroviral therapies work well using direct observed therapy in the controlled setting of a clinical trial (i.e., they are efficacious), once applied to a free-living population, the drug dosing regimen may be too difficult for patients to follow in order to be effective. Finally, efficiency evaluates the costs and benefits of a medical intervention.
What Are the Appropriate Statistical Tests?
Once the appropriate design is determined for a particular study question, it is important to consider the appropriate statistical tests that must be (or have been) performed on the data collected. This is relevant whether one is reviewing a scientific article or planning a clinical study. To begin, we will look at terms and calculations that are used primarily to describe measures of central tendency and dispersion. These measures are important in understanding key aspects of any given dataset.
Measures of Central Tendency
There are three commonly referred to measures of central location: mean, median, and mode. The arithmetic mean or average is calculated by summing the values of the observations in the sample and then dividing the sum by the number of observations in the sample. This measure is frequently reported for continuous variables: age, blood pressure, pulse, body mass index (BMI), to name a few. The median is the value of the central observation after all of the observations have been ordered from least to greatest. It is most useful for ordinal or non-normally distributed data. For data sets with an odd number of observations, we would determine the central observation with the following formula:

For datasets with an even number of observations, we would select the case that was the average of the following observations' values:

The mode is the most commonly occurring value among all the observations in the dataset. There can be more than one mode. The mode is most useful in nominal or categorical data. Typically no more than two (bimodal) are described for any given dataset.
Example 1: A patient records his systolic blood pressure every day for one week. The values he records are as follows: Day 1: 98 mmHg, Day 2: 140 mmHg, Day 3: 130 mmHg, Day 4: 120 mmHg, Day 5: 130 mmHg, Day 6: 102 mmHg, Day 7: 160 mmHg.
The arithmetic mean or average for these 7 observations is calculated as follows:

Table 3: Advantaged and Disadvantages of Measure of Central Tendency and Dispersion*

Measures of Dispersion
Measures of dispersion or variability provide information regarding the relative position of other data points in the sample. Such measures include the following: range, inter-quartile range, standard deviation, standard error of the mean (SEM), and the coefficient of variation.
Range is a simple descriptive measure of variability. It is calculated by subtracting the lowest observed value from the highest. Using the blood pressure data in example 1, the range of blood pressure would be 160 mmHg minus 98 mmHg or 62 mmHg. Often given with the median (i.e., for non-normally distributed data) is the interquartile range, which reflects the values for the observations at the 25th and 75th percentiles of a distribution.
The most commonly used measures of dispersion include variance and its related function, standard deviation, both of which provide a summary of variability around the mean. Variance is calculated as the sum of the squared deviations divided by the total number of observations minus one:

The standard deviation is the square root of the variance. Table 4 presents calculations of variance and standard deviation for the systolic blood pressures given in example 1.
Table 4: Example of a Standard Deviation Calculation

The coefficient of variation (CV) is a measure that expresses the SD as a proportion of the mean:

This measure is useful if the clinician wants to compare 2 distributions that have means of very different magnitudes. From the data provided in example 1, the coefficient of variation would be calculated as follows:

The standard error of the mean (SEM) measures the dispersion of the mean of a sample as an estimate of the true value of the population mean from which the sample was drawn. It is related to, but different from, the standard deviation. The formula is as follows:

Using the data from example 1, the SEM would be:

SEM can be used to describe an interval within which the true sample population mean lies, with a given level of certainty. Which measure of dispersion to use is dependent on the study purpose. Table 3 provides some information which may facilitate the selection of the appropriate measure or measures.
Comparing Central Tendencies with Respect to Dispersions (Error Terms)
Once central tendency and dispersion are measured, it follows that a comparison between various groups (e.g., level of systolic blood pressure among persons taking ACE-Inhibitors versus beta-blockers) is desired. If working with continuous variables that are normally distributed, the comparison is between means. The first step is to simply look at the means and see which is larger (or smaller) and how much difference lies between the two. This step is the basis of deductive inference. In comparing the means, and, preferably before calculating any p-values, the clinician or investigator must answer the question: is the observed difference clinically important? If the magnitude of the observed difference is not clinically important, then the statistical significance becomes irrelevant in most cases. If the observed difference is clinically important, even without statistical significance, the finding may be important and should be pursued (perhaps with a larger and better powered study; Table 5 ).
Clinical Versus Statistical Significance and Possible Conclusions

Once a deductive inference is made on the magnitude of the observed differences, statistical inference follows to validate or invalidate the conclusion from the deductive inference. To illustrate: if two people each threw one dart at a dartboard, would one conclude that whoever landed closer to the center was the more skilled dart thrower? No. Such a conclusion would not be reasonable even after one game or one match as the result may be due to chance. Concluding who is a better player would have to be based on many games, against many players, and over a period of time. There are many reasons for inconsistencies (good day, bad day, etc.), but they all boil down to variance. For a player to be classified as a “good player,” he/she has to be consistently good over time.
Because in clinical research we rely on a sample of the patient population, variance is a key consideration in the evaluation of observed differences. The observed difference between exposed and unexposed groups can be large, but one must consider how it stands next to the variation in the data. Since these parameters are highly quantifiable, the probability that the means are different (or similar) can be calculated. This process takes place in a statistical method called analysis of variance (ANOVA). The details of this process are beyond the scope of this chapter; nevertheless, ANOVA is a fundamental statistical methodology and is found in many texts and is performed by many statistical software packages. In essence, the ANOVA answers the question: are differences between the study groups' mean values substantial relative to the overall variance (all groups together)? It is important to note that even though ANOVA reveals statistically significant differences, the ANOVA does not indicate between which groups the difference exists. Therefore, further analysis with multiple comparison tests must be performed to determine which means are significantly different. Portney and Watkins (19) provide a good overview of these procedures.
In the special case where one and only one comparison can be made, the t-test can be done. It was developed to be a shortcut comparison of only two means between groups with small sample sizes (less than 30). If used for more than one comparison or when more than one comparison is possible, the t-tests do not protect against Type 1 error at the assumed level of tolerance for Type 1 error (usually α = 0.05).
Probability: Fundamental Concepts in Evidence-Based Medicine
Armed with a basic understanding of algebra and user-friendly statistical software, most clinicians and clinical researchers can follow the cookbook method of statistical inference. Problems quickly arise because the vast majority of medical research is not designed as simply as the examples given in basic statistics textbooks nor analyzed as simply as the shortcut methods often programmed beneath the layers of menus in easy-to-use software. Violations of assumptions that are necessary for a classic statistical method to be valid are more the rule than the exception. However, avoiding the misinterpretation of statistical conclusions does not require advanced mastery of the mathematics of probability at the level of calculus. An effort to understand, at least qualitatively, how to measure the degree of belief that an event will occur will go a long way in allowing non-mathematicians to make confident conclusions with valid methods.
Two practical concepts should be understood up front: first, understanding that every probability, or rate, has a quantifiable uncertainty that is usually expressed as a range or confidence interval. Second, that comparing different rates observed between two populations, or groups, must be done relative to the error terms. This is the essence of statistical inference.
Probability Distributions:
The final rate of an event that is measured, as the size of the sample being measured grows to include the entire population, is the probability that any individual in the population will experience the event. For example, one analyzes a database of heart transplant patients who received hearts from donors over the age of 35 to determine the rate of cardiac death within a 5-year post-transplant follow-up period (20) . If the first patient in the sample did not survive the study period, the sample of this one patient gives an estimated cardiac death rate of 100%. No one would accept an estimate from a sample of one. However, as the sample size increases, the event rate will migrate towards truth. If the next patient in the database is a survivor, the cardiac death rate falls to 50%. Once the entire population represented in the database is included in the sample (n=26), it is observed that 7 experienced cardiac death for a final cardiac death rate of 27%. When written as a probability, one can say that the probability is 0.27 that any single participant randomly sampled from this database will be recorded as having a cardiac death within 5 years of receiving a heart transplant. It may be more relevant to use the data to predict that the next patient seen in clinic and added to the database will have a probability of 0.27 of experiencing cardiac death within 5 years. The illustration just described is that of a binomial probability. That is, the outcome is one of two possible levels (binary): survival or death.

For this example, the SE is calculated by:

The Z score for a two sided 95% confidence interval (CI) is 1.96, so the range of the CI is calculated by lower 95% CI = 0.27 − 1.96 × 0.087 = 0.011 and upper 95% CI = 0.27 + 1.96 × 0.087 = 0.440, respectively. These yield the range (.011, .440). Thus, if this observation were repeated 100 times in similar populations of the same sample size, 95 of the sampled death rates would fall between .011 and .440.
Evaluating Diagnostic and Screening Tests
In order to understand disease etiology and to provide appropriate and effective health care for persons with a given disease, it is essential to distinguish between persons in the population who do and do not have the disease of interest. Typically, we rely on screening and diagnostic tests that are available in medical facilities to provide us information regarding the disease status of our patients. However, it is important to assess the quality of these tests in order to make reasonable decisions regarding their interpretation and use in clinical decision-making (1) . In evaluating the quality of diagnostic and screening tests, it is important to consider the validity (i.e. sensitivity and specificity) as well as the predictive value (i.e. positive and negative predictive values) of the test.
Sensitivity is the probability (Pr) that a person will test positive (T+) given that they have the disease (D+). Specificity is the probability (Pr) that a person will test negative (T−) given that they do not have the disease (D−). These are conditional probabilities. The result in question is the accuracy of the test, and the condition is the true, yet unknown, presence or absence of the disease. Sensitivity and specificity are properties of the screening test, and, like physical properties, follow the test wherever it is used. They can be useful in determining the clinical utility of the test (as a screening tool vs. a diagnostic tool) as well as comparing new tests to existing tests. They are written mathematically as:

It is more common for sensitivity and specificity to be expressed from a 2×2 contingency table (Table 6) as follows:

Table 6: 2×2 Contigency Table – Test Characteristics

These parameters quantify the validity of a test when it is evaluated in a population that represents the spectrum of patients in whom it would be logical and clinically useful to use the test. The most obvious limitation of evaluating a screening test is identifying an optimal gold standard to determine the disease status. In the evaluation of new screening tests, existing tests are often used as the gold standard. Disagreement or poor sensitivity and specificity of the new test could mean that the new test does not work as well as, or that it is actually superior to, the existing test. A histological test from a biopsy is the least disputable gold standard. Nonetheless, the limitation with regards to the gold standard is unavoidable and must be recognized in the continuous evaluation of clinical screening and diagnostic testing.
In a study to evaluate bedside echocardiography by emergency physicians to detect pericardial effusion, 478 eligible patients were evaluated for the condition both by the emergency department physician and by the cardiologist (who had the clinical responsibility to make the diagnosis); the cardiologist's finding was used as the gold standard (21) .
An excerpt of the results is shown in Table 7 . From the data presented in the table, the following can be calculated:

Table 7: 2×2 Contingency Table for Pericardial Effusion Study

Since the sensitivity and specificity are like physical properties of the test, we can determine the portion of TP and TN regardless of the prevalence of the disease in the population studied. For example, if we had recruited 100 patients with pericardial effusion and 100 matching participants without pericardial effusion, the resulting table would yield the same rates of TP, TN, and identical values for sensitivity and specificity (Table 8) .

Table 8: 2×2 Contingency Table for Pericardial Effusion Example

Positive predictive value (PPV) is the probability (Pr) that the disease is truly present (D+) given that the test result is positive (T+). Negative predictive value (NPV) is the probability that the disease is truly absent (D−) given that the test result is negative (T−). Generally speaking, patients (and their physicians) are more concerned with these probabilities. These are also conditional probabilities. These parameters are written mathematically as:
PPV = Pr(D + |T + ), and NPV = Pr(D − |T − ). As with sensitivity and specificity, it may be more common to see the algebraic expressions:

The question is whether or not the individual patient's test result is true. Unlike sensitivity and specificity, PPV and NPV are dependent upon the prevalence of the disease in the population. The example below further illustrates this point.

Figure 1 shows PPV and NPV over the entire range of possible prevalence with sensitivity and specificity fixed at the values in the illustration. PPV and NPV are used to make clinical decisions concerning an individual patient based on the population from which the patient comes. As prevalence increases, PPV increases and NPV decreases. Thus, in populations where disease prevalence is high, there will be greater confidence that a positive test result is a true positive, and increased suspicion that a negative test result is a false negative. The reverse is true in populations where the disease prevalence is low (e.g. rare disease).

Figure 1: Effect of Prevalence on Positive and Negative Predictive Values
Diagnostic and screening tests, and their related sensitivities, specificities, PPVs and NPVs, facilitate the clinician's classification of a patient with regard to a specific disease status. The ultimate goal of the diagnostic process is to establish a diagnosis with sufficient confidence to justify treatment or to exclude a diagnosis with sufficient confidence to justify non-treatment (22) . In the process of determining a diagnosis (or not), the test results for a given disease should be kept within the context of the probability of disease prior to receiving results. Bayesian logic is the understanding of conditional probability which is expressed mathematically in Baye's Theorem. The theorem “indicates that the result of any diagnostic test alters the probability of disease in the individual patient because each successive test result reclassifies the population from which the individual comes” (22) .
Common Measures of Association and Statistical Tests
Measures of association are summary statistics that estimate the risk of an outcome or disease for a given exposure between two groups. Two frequently reported measures are the odds ratio and the relative risk. The odds ratio (OR) is calculated from a case-control study where the participants were selected by their outcome and then studied to determine exposure. Because the participants are selected on outcome, the case-control study reveals the prevalence of exposure among cases and controls. In case-control studies we calculate odds ratios because it is often a good estimate of the relative risk. Odds are the probability of an event occurring divided by the probability of the event not occurring. The OR ratio compares the odds of being exposed given a participant is a case ( Table 9 : a / a+c / c / a+c = a/c) relative to the odds of control participants being exposed (b/b+d / d / b+d = b/d). Using algebra to re-arrange the formula, the OR can be calculated as (Table 9) :

Table 9: Cell Naming Scheme for Doing Calculations from a 2 × 2 Table

Table 10: Diagram of Observed Frequencies Extracted for Odds Ratio Example

The relative risk or risk ratio (RR) is calculated from a cohort study where exposed and non-exposed participants are followed over time and the incidence of disease is observed. Because the hallmark of a cohort study is following a population over time to identify incident cases of disease, the cohort is screened to assure that no participant enrolled in the study has already experienced the outcome or disease event. Then, the cohort is followed for a specific period of time, and the incidence of events for the exposed and unexposed groups is measured. The relative risk can also be used to analyze clinical trial data. The relative risk (RR) is calculated from the labeled 2×2 table (Table 9) using the formula:

Table 11: Observed Frequencies Extracted from Relative Risk Example*

Both the OR and RR have confidence intervals (CI) as a measure of uncertainty. The method is similar to the one used for the binomial probability distribution. If a 95% CI excludes the value one (1) , then the ratio is significant at the level of p<0.05. A test of independence, as a category of methods, tests the hypothesis that the proportion of an outcome is independent of the grouping category. The alternate hypothesis, the conclusion made when the p-value is significant (p<0.05), is that the disease or outcome is more common among the exposed or unexposed group.
Chi-square tests are used to determine the degree of belief that an observed frequency table could have occurred randomly by comparing it to an expected frequency table. The expected frequency table is derived based on the assumption that the row and column totals are true as observed and fixed. The most commonly used chi-square test is the Pearson's chi-square test. This is used to analyze a frequency table with two rows and two columns. When the table is not symmetrical or is of dimensions other than 2-by-2, the method is still valid, and when used is called the Cochran's chi-square test. At the very least, the largest observed difference is significant if the table is significant. If the overall table is significant, this global significance can allow stratified sub-analyses of the individual comparisons of interest. It can also be helpful to look at the contribution to the chi-square test statistic by each cell and conclude that the largest of these cells are where the observed frequencies most deviated from the expected frequencies.

Table 12: Components for Calculating a 95% Confidence Interval Around Measures of Association

Tests of proportional disagreement are for paired data, either repeated measures in the same participants or participants matched on demographic factors then given different exposures and followed to compare outcomes. The best known of the tests of proportional disagreement is the McNemar's chi-square test. The outcome of paired data falls into four observations (++, −–, +–, −+). McNemar's test focuses on the discordant cells (+–, −+) and tests the hypothesis that the disagreement is proportional between the two groups. If when the outcome disagrees, the disagreement is more frequently −+ than +–, then we know that more pairs are improving or having better outcomes. A relatively new application of tests for paired data is the Combined Quality Improvement Ratio (CQuIR), which uses the McNemar's chi-square test as the basis, but combines participants with repeated measures and case-control matched pairs into one large database of analyzable pairs. This process maximizes the statistical power available from the population ( 25 , 26 ). Additionally, the ratio of discordant pairs (−+/+−) shows whether or not the disagreement is more often toward improvement. Included in the tests of disproportion is the Kappa statistic of agreement. The Kappa statistic evaluates the concordant cells (++ and −−) to conclude whether or not the agreement has enough momentum to be reproducible.
Thus far, we have used examples for analyses from observational studies. Experimental studies or clinical trials are analyzed in much the same manner. In clinical trials, patients are followed until some outcome is observed in the planned study period; these are incidence studies. As incidence studies, the RR will be the measure of association tested for statistical significance. Additionally, many clinical trials lend themselves to straightforward analyses with chi-square tests, ANOVA, or other methods that result only in a p-value. Table 13 summarizes common methods used to analyze healthcare data.
Measures of Association and Statistical Tests Commonly Used to Analyze Healthcare Data

For one example, we review the results of a trial of the beta-blocker, bucindolol, used in patients with advanced chronic heart failure (CHF) (27) . While it is accepted that beta-blockers reduce morbidity and mortality in patients with mild to moderate CHF, these investigators enrolled 2708 patients designated as New York Heart Association (NYHA) class III or IV to test the efficacy of the beta-blocker in reducing morbidity and mortality in patients with high baseline severity. The primary outcome of interest was all-cause-mortality, which, being a relatively rare event, drove the sample size requirement to 2800 in order to statistically detect a clinically significant difference of 25%. Once enrolled, patients were randomly assigned to receive either placebo or the beta-blocker, and neither the patient nor the physician knew to which treatment the patient was assigned. This study was stopped after the seventh interim analysis due to the accruing evidence of the usefulness of beta-blockers for CHF patients from other studies. At the time the study was stopped, there was no difference in mortality between the two groups (33% in the placebo group vs. 30% in the beta-blocker group, p=0.16). After the follow-up data was completed, adjustments for varying follow-up time could be made. The adjusted difference in mortality rate was still not significant (p=0.13). However, a sub-analysis of the secondary endpoint of cardiac death did yield a significant hazard ratio (HR) of 0.86 with a 95% CI of 0.74 to 0.99. This HR being less than the value 1 means that the beta-blocker was protective against cardiac death in the follow-up period. The CI not including the value 1 leads to the conclusion that this HR is statistically significant at the level of p<0.05. This secondary analysis is consistent with the decision of the study group to stop the trial early.
This concludes Part I of the series. In the next issue of The Ochsner Journal , we will present Part II which includes discussion of the significance of the study results, relevance of the results in clinical practice, and study limitations.
Table 14: Classification of Random Error

- Gordis L. Epidemiology. Philadelphia: W.B. 1996 Saunders Company. [ Google Scholar ]
- Rosner B. Fundamentals of Biostatistics. 1990 Boston: PWS-Kent Publishing Company. [ Google Scholar ]
- Conover W. J. Practical Nonparametric Statistics. 1971 New York: John Wiley & Sons, Inc. [ Google Scholar ]
- MacMahon B., Pugh T. F. Epidemiology: Principles and Methods. 1970 Boston: Little, Brown. [ Google Scholar ]
- Hennekens C. H., Buring J. E. Epidemiology in Medicine. Mayrent SL (ed) 1987 Boston: Little, Brown and Company. [ Google Scholar ]
- Glantz S. A. Primer of Biostatistics, 5th Edition. 2002 Ohio: McGraw-Hill Appleton & Lange. [ Google Scholar ]
- St Leger A. S., Cochrane A. L., Moore F. Factors associated with cardiac mortality in developed countries with particular reference to the consumption of wine. Lancet. 1979; 1 (8124):1017–1020. [ PubMed ] [ Google Scholar ]
- Kwon H. J., Cote T. R., Cuffe M. S., Kramer J. M., Braun M. M. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003; 138 (10):807–811. [ PubMed ] [ Google Scholar ]
- Ford E. S., Giles W. H., Dietz W. H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002; 287 (3):356–359. [ PubMed ] [ Google Scholar ]
- Bazzano L. A., He J., Muntner P., Vupputuri S., Whelton P. K. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003; 138 (11):891–897. [ PubMed ] [ Google Scholar ]
- Vupputuri S., Batuman V., Muntner P. Effect of blood pressure on early decline in kidnery function among hypertensive men. Hypertension. 2003; 42 (6):1144–1149. [ PubMed ] [ Google Scholar ]
- Kannel W. B., Dawber T. R., Kagan A., Revotskie N., Stokes J., 3rd Factors of risk in the development of coronary heart disease - six year follow-up experience. The Framingham Study. Ann Intern Med. 1961; 55 :33–50. [ PubMed ] [ Google Scholar ]
- Vasan R. S., Larson M. G., Leip E. P. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001; 358 (9294):1682–1686. [ PubMed ] [ Google Scholar ]
- O'Donnell C. J., Larson M. G., Feng D. Genetic and environmental contributions to platelet aggregation: the Framingham Heart study. Circulation. 2001; 103 (25):3051–3056. [ PubMed ] [ Google Scholar ]
- D'Agostino R. B., Russell M. W., Huse D. M. Primary and subsequent coronary risk appraisal: New results from the Framingham study. Am Heart J. 2000; 139 (2 Part 1):272–281. [ PubMed ] [ Google Scholar ]
- The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989; 129 (4):687–702. [ PubMed ] [ Google Scholar ]
- Harjai K. J., Boulos L. M., Smart F. W. Effects of caregiver specialty on cost and clinical outcomes following hospitalization for heart failure. Am J Cardiol. 1998; 82 (1):82–85. [ PubMed ] [ Google Scholar ]
- Strandberg T. E., Pitkala K., Berglind S. Multifactorial cardiovascular disease prevention in patients aged 75 years and older: a randomized controlled trial: Drugs and Evidence Based Medicine in the Elderly (DEBATE) Study. Am Heart J. 2001; 142 (6):945–951. [ PubMed ] [ Google Scholar ]
- Portney L. G., Watkins M. P. Foundations of Clinical Research Applications to Practice. 2nd edition. 2000 Upper Saddle River, NJ: Prentice-Hall Health. [ Google Scholar ]
- Mehra M. R., Ventura H. O., Chambers R. B., Ramireddy K., Smart F. W., Stapleton D. D. The prognostic impact of immunosuppression and cellular rejection on cardiac allograft vasculopathy: time for a reappraisal. J Heart Lung Transplant. 1997; 16 (7):743–757. [ PubMed ] [ Google Scholar ]
- Mandavia D. P., Hoffner R. J., Mahaney K., Henderson S. O. Bedside echocardiography by emergency physicians. Ann Emerg Med. 2001; 38 (4):377–382. [ PubMed ] [ Google Scholar ]
- Katz D. L. Clinical Epidemiology and Evidence-Based Medicine: Fundamental Principles of Clinical Reasoning and Research. 2001 Thousand Oaks, CA: Sage Publications, Inc. [ Google Scholar ]
- Chen L., Chen M. H., Larson M. G., Evans J., Benjamin E. J., Levy D. Risk factors for syncope in a community-based sample (the Framingham Heart study) Am J Cardiol. 2000; 85 (10):1189–1193. [ PubMed ] [ Google Scholar ]
- Kim K. S., Owen W. L., Williams D., Adams-Campbell L. L. A Comparison between BMI and conicity index on predicting coronary heart disease: the Framingham Heart Study. Ann Epidemiol. 2000; 10 (7):424–431. [ PubMed ] [ Google Scholar ]
- Chambers R. B., Krousel-Wood M. A., Re R. N. Combined quality improvement ratio: a method for a more robust evaluation of changes in screening rates. Jt Comm J Qual Improv. 2001; 27 (2):101–106. [ PubMed ] [ Google Scholar ]
- Krousel-Wood M. A., Chambers R. B., Re R. N., Nitzkin P. R., Cortez L. M. Application of the combined quality improvement ratio in the evaluation of a quality improvement activity in a managed care organization. Am J Med Quality. 2003; 18 :117–121. [ PubMed ] [ Google Scholar ]
- The beta-blocker evaluation of survival trial investigators: a trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001; 344 (22):1659–1667. [ PubMed ] [ Google Scholar ]
Diseases & Conditions
Easy-to-understand answers about diseases and conditions
Find diseases & conditions by first letter
Symptom checker.
Find out what could be causing your symptoms and when to seek care.

Clinical trials
Search for clinical trials by disease, treatment, or drug name.
Connect to support groups
Share your experiences and find support in our online communities.

The right answers, the first time
Mayo Clinic experts solve the world’s toughest medical problems — one patient at a time.
Double your impact on fighting cancer
Make a gift before July 31 and it can go twice as far to fight cancer.
Part 1. Overview Information
National Institutes of Health ( NIH )
R03 Small Grant Program
- April 4, 2024 - Overview of Grant Application and Review Changes for Due Dates on or after January 25, 2025. See Notice NOT-OD-24-084 .
- August 31, 2022 - Implementation Changes for Genomic Data Sharing Plans Included with Applications Due on or after January 25, 2023. See Notice NOT-OD-22-198 .
- August 5, 2022 - Implementation Details for the NIH Data Management and Sharing Policy. See Notice NOT-OD-22-189 .
See Section III. 3. Additional Information on Eligibility .
The goal of the Grants for Early Medical/Surgical Specialists' Transition to Aging Research (GEMSSTAR) program is to provide support for early-career physician-scientists trained in medical or surgical specialties and early-career dentist-scientists to launch careers as future leaders in aging- or geriatric-focused research. In support of the program's goal, this GEMSSTAR notice of funding opportunity (NOFO) invites applications that propose to conduct transdisciplinary aging research that will yield pilot data and experience for subsequent aging research projects. The GEMSSTAR program encourages candidates to seek out a supportive research environment to further the program's objectives of fostering the development of early-career physician- and dentist-scientists in aging- or geriatric-focused research, particularly as it applies to their clinical specialty/discipline. Toward this end, GEMSSTAR candidates should include a Professional Development Plan that reflects this supportive research environment. In selecting GEMSSTAR awardees, the National Institute on Aging (NIA) will consider the extent to which a candidate's environment is supportive of aging- or geriatric-focused research.
October 01, 2024
| Application Due Dates | Review and Award Cycles | ||||
|---|---|---|---|---|---|
| New | Renewal / Resubmission / Revision (as allowed) | AIDS - New/Renewal/Resubmission/Revision, as allowed | Scientific Merit Review | Advisory Council Review | Earliest Start Date |
| November 01, 2024 | November 01, 2024 | Not Applicable | March 2025 | May 2025 | July 2025 |
All applications are due by 5:00 PM local time of applicant organization's time zone.
Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date.
No late applications will be accepted for this Notice of Funding Opportunity (NOFO).
Not Applicable
It is critical that applicants follow the instructions in the Research (R) Instructions in the How to Apply - Application Guide , except where instructed to do otherwise (in this NOFO or in a Notice from NIH Guide for Grants and Contracts ).
Conformance to all requirements (both in the How to Apply - Application Guide and the NOFO) is required and strictly enforced. Applicants must read and follow all application instructions in the How to Apply - Application Guide as well as any program-specific instructions noted in Section IV. When the program-specific instructions deviate from those in the How to Apply - Application Guide , follow the program-specific instructions.
Applications that do not comply with these instructions may be delayed or not accepted for review.
There are several options available to submit your application through Grants.gov to NIH and Department of Health and Human Services partners. You must use one of these submission options to access the application forms for this opportunity.
- Use the NIH ASSIST system to prepare, submit and track your application online.
- Use an institutional system-to-system (S2S) solution to prepare and submit your application to Grants.gov and eRA Commons to track your application. Check with your institutional officials regarding availability.
- Use Grants.gov Workspace to prepare and submit your application and eRA Commons to track your application.
Part 2. Full Text of Announcement
Section i. notice of funding opportunity description.
With the growing number of older adults with complex health challenges, there is an equally growing need for medical, surgical, and dental clinician-scientists with training and experience in research focused on optimizing the care of older patients. A pivotal point in the development of clinician-scientists is the completion of specialty training and the first faculty appointment. At this early stage, limited research experience and publication record preclude competitiveness for many kinds of advanced research opportunities. This transition stage may be particularly difficult for physicians or dentists focusing on aging-related aspects of their specialty/discipline, as multidisciplinary research entails training that is rarely available in conventional programs. Thus, mechanisms that allow these clinicians to initiate research projects and establish research track records are critical for cultivating the next generation of clinician-scientists at the interface of aging and medical, dental, and surgical specialties.
The goal of the GEMSSTAR program is to provide support for early-career physician-scientists trained in medical or surgical specialties and early-career dentist-scientists for small, transdisciplinary research projects on questions relevant to aging and/or the aged to launch careers as future leaders in aging- or geriatric-focused research. This GEMSSTAR NOFO invites applications proposing to conduct transdisciplinary aging research that will yield pilot data and experience for subsequent aging research projects. The GEMSSTAR program encourages candidates to seek out a supportive research environment that will further the program's objectives of fostering the development of early-career physician- and dentist-scientists in aging- or geriatric-focused research, particularly as it applies to their clinical specialty/discipline. In selecting GEMSSTAR awardees, NIA will consider the extent to which a candidate's environment is supportive of aging- or geriatric-focused research.
Program Description
The GEMSSTAR program is intended to support an early-career physician's or dentist's first independent research project. The program also provides an opportunity for physicians and dentists who are funded in non-aging-related fields to refocus their research efforts on aging- or geriatrics-related topics. Thus, the program seeks applicants who aspire to continue or shift their research focus to bridge their specialty/discipline and the clinical care of older adults.
The GEMSSTAR program provides two years of funding. Applicants should emphasize integration of gerontologic or geriatric research with the candidate’s clinical specialty/discipline. Proposed projects may involve pilot or feasibility studies, secondary analyses of existing data, development of research methodology, development of new research technology, or other similar approaches. Projects may span the breadth of scientific domains, including basic, translational, clinical, genetic, or epidemiologic science. Human subjects, animal models, and in vitro systems are all acceptable as appropriate to the research questions. Projects should be appropriate to the background and level of experience of the applicant. Potential research topics may include, but are not limited to, the following:
- Characterization of an aging-related disease, condition, syndrome, or phenomenon relevant to a clinical specialty/discipline
- Elucidation of mechanisms underlying specialty/discipline-related diseases in older age
- Identification of predictors and/or outcomes of specialty/discipline-related interventions specific to older populations
- Pilot investigation of a specialty/discipline-related intervention in older adults
- Development of strategies to address and/or integrate important complexities common in older patients typically seen within a clinical specialty/discipline, such as multiple chronic conditions; polypharmacy; multispecialty guideline integration; and preservation of function, cognition, and independence
- Multidisciplinary care strategies, such as palliative care, to improve outcomes in older patients within or across care settings
- Specific diagnostic, management, and/or decision-making and communication strategies pertaining to older adults with Alzheimer's disease (AD) or AD-related dementias (ADRD) within or across medical and/or surgical specialties and primary care/geriatrics
- Strategies for screening, primary prevention, and/or secondary prevention of specialty/discipline-related diseases in older adults
- Initial development of tools to assess the risk and/or prognosis of a specialty/discipline-related disease and/or interventions in older adults
Please refer to Section III. Eligibility Information, Eligible Individuals (Program Director/Principal Investigator) for clarification on eligibility. It is expected that applicants will have expertise in their clinical specialty/discipline, but may be less experienced in geriatric/gerontologic science or in other areas. As such, applicants should include participation of a senior collaborator with complementary expertise in aging-related research and, if needed, other collaborators and/or consultants in additional areas appropriate to the proposed project.
Geriatricians proposing a research project that integrates their geriatrics expertise with a specific clinical problem that is typically embraced by another specialty should involve a senior collaborator with expertise in that clinical problem as it relates to older patients. For example, a geriatrician researcher proposing a project focused on chronic kidney disease is encouraged to involve a senior nephrologist or other relevant specialist.
In addition to the research project, candidates must also include a Professional Development Plan that describes an individualized plan to garner resources, activities, collaborations, and/or didactic or practical experiences concurrent with the R03 research project that will 1) lead to enhanced knowledge and skills in aging/geriatrics science, and 2) increase the applicant's likelihood of successful completion of their proposed GEMSSTAR project. This plan would complement the GEMSSTAR R03 award and would need to be supported independent of the R03 research plan and funding.
More details about the Professional Development Plan and other details can be found on the NIA GEMSSTAR webpage . Prospective applicants are strongly encouraged to visit this webpage for answers to frequently asked questions and to learn more about the GEMSSTAR program.
Clinical Research
NOTE TO APPLICANTS CONSIDERING A CLINICAL TRIAL: The limited time and budget provided by this NOFO will constrain the types of clinical trials that can be proposed. In general, only mechanistic trials or small pilot trials are likely to be feasible. Applicants considering a clinical trial are highly encouraged to contact NIA program staff early to discuss the feasibility of doing so. Refer also to the NIH's policies and requirements for clinical trials . Investigators proposing NIH-defined clinical trials may refer to the Research Methods Resources website for information about developing statistical methods and study designs.
Clinical Research Operations Management System
NIA uses a central resource to NIA staff and extramural investigators to facilitate/support the conduct and management of clinical research. NIA Clinical Research Operations & Management System (CROMS) is a comprehensive data management system to support the business functions, management, and oversight responsibilities of NIA grants that support the conduct of clinical research with human subjects. NIA investigators of grants, contracts, and cooperative agreements that are active as of July 1, 2021, including clinical trials funded as pilots, exploratory studies, or other projects through this Consortium, and support human subjects research as defined by the DHS HHS OHRP regulations at 45 CFR 46 will be required to interact with and use existing and future components of CROMS as required by NIA throughout the lifecycle of the grant, as described in NOT-AG-23-017 . Data to be submitted to NIA CROMS includes those elements reported in the standard NIH requirement annual progress report (GPS 4.1.15.7). Details regarding the standard operating procedures for CROMS can be found on the NIA CROMS website .
When applicable, all NIA grantees must ensure:
1. The study’s Informed Consent Document (ICD) lists “The National Institutes of Health (NIH) and its authorized representatives” as one of the organizations that may look at or receive copies of information in participants’ study records. According to DHS HHS OHRP 45 CFR 46 §46.116 , all ICDs must contain “A statement describing the extent, if any, to which confidentiality of records identifying the participant will be maintained.” If using the NIA informed consent template , please see Section 6: Statement of Confidentiality.
2. An assigned NIH ClinicalTrials.gov identifier (NCT number) is reported in its respective CROMS study record within three months after assignment, and the reporting of final enrollment data to CROMS is consistent with final enrollment data reported in ClinicalTrials.gov.
NIA's Commitment to Inclusivity in Research Involving Human Subjects
NIA is committed to supporting and conducting research on aging that improves the health and well-being of all people. Therefore, NIA will prioritize the advancement of science that represents, in terms of race, ethnicity, sex, age, and comorbidity, the population affected by the condition being studied. Applicants should ensure as applicable that they 1) include proposed planned enrollment tables identifying the population(s) affected by the disease/condition, and 2) address the NIH Inclusion Policies for Research Involving Human Subjects and NIH-designated Populations with Health Disparities , as appropriate; as well as other populations that experience health disparities.
See Section VIII. Other Information for award authorities and regulations.
Section II. Award Information
Grant: A financial assistance mechanism providing money, property, or both to an eligible entity to carry out an approved project or activity.
The OER Glossary and the How to Apply - Application Guide provide details on these application types. Only those application types listed here are allowed for this NOFO.
Optional: Accepting applications that either propose or do not propose clinical trial(s).
Need help determining whether you are doing a clinical trial?
Funds Available: $3.8 million in fiscal year 2025
Anticipated Number of Awards: 15 general awards and 4 Alzheimer’s disease (AD) or AD-related dementias (ADRD) awards.
Application budgets are limited to $125,000 in direct costs per year.
Indirect costs are those for a common or joint purpose across more than one project and that cannot be easily separated by project. Learn more at 45 CFR 75.414 , Indirect Costs.
To charge indirect costs applicants can select one of two methods:
Method 1 – Approved rate. The applicant organization currently has an indirect cost rate approved by the applicant organization’s cognizant federal agency.
Method 2 – De minimis rate. Per 45 CFR 75.414(f) , if the applicant organization has never received a negotiated indirect cost rate or is awaiting approval of an indirect cost proposal, they may elect to charge a de minimis rate. If choosing this method, costs included in the indirect cost pool must not be charged as direct costs.
This rate is 10% of modified total direct costs (MTDC). See 45 CFR 75.2 for the definition of MTDC. Organizations can use this rate indefinitely.
The project period is limited to 2 years.
NIH grants policies as described in the NIH Grants Policy Statement will apply to the applications submitted and awards made from this NOFO.
Section III. Eligibility Information
1. eligible applicants eligible organizations higher education institutions public/state controlled institutions of higher education private institutions of higher education the following types of higher education institutions are always encouraged to apply for nih support as public or private institutions of higher education: hispanic-serving institutions historically black colleges and universities (hbcus) tribally controlled colleges and universities (tccus) alaska native and native hawaiian serving institutions asian american native american pacific islander serving institutions (aanapisis) nonprofits other than institutions of higher education nonprofits with 501(c)(3) irs status (other than institutions of higher education) nonprofits without 501(c)(3) irs status (other than institutions of higher education) for-profit organizations small businesses for-profit organizations (other than small businesses) local governments state governments county governments city or township governments special district governments indian/native american tribal governments (federally recognized) indian/native american tribal governments (other than federally recognized) federal governments eligible agencies of the federal government u.s. territory or possession other independent school districts public housing authorities/indian housing authorities native american tribal organizations (other than federally recognized tribal governments) faith-based or community-based organizations regional organizations foreign organizations non-domestic (non-u.s.) entities (foreign organizations) are not eligible to apply. non-domestic (non-u.s.) components of u.s. organizations are not eligible to apply. foreign components, as defined in the nih grants policy statement , are allowed. required registrations applicant organizations the applicant organization must be registered in sam.gov, grants.gov, and era commons before submitting an application. while applicants can review the requirements and start developing applications before registrations are complete, applicants should complete these steps early. failure to complete required registrations before the due date is not a valid reason for a late submission. please reference nih grants policy statement section 2.3.9.2 electronically submitted applications for more information. system for award management (sam) – applicant organizations must have an active account with sam.gov. this includes having a unique entity identifier (uei). sam.gov registration can take 6 weeks or longer. begin that process today. to register, go to sam.gov entity registration and select get started. select the entity registration checklist on the same page to view a list of the information needed to need to register. organizations must maintain an active registration, which requires renewal at least annually. the renewal process may require as much time as the initial registration. after registering in sam, organizations can register with grants.gov and era commons. nato commercial and government entity (ncage) code – foreign organizations must obtain an ncage code (in lieu of a cage code) in order to register in sam. unique entity identifier (uei) - a uei is issued as part of the sam.gov registration process. the same uei must be used for all registrations, as well as on the grant application. era commons - once the unique organization identifier is established, organizations can register with era commons in tandem with completing their grants.gov registrations; all registrations must be in place by time of submission. era commons requires organizations to identify at least one signing official (so) and at least one program director/principal investigator (pd/pi) account in order to submit an application. make sure the organization uses the same uei on the application, in sam.gov, and in era commons. grants.gov – applicant organizations must also have an active account with grants.gov. see step-by-step instructions at the grants.gov quick start guide for applicants . program directors/principal investigators (pd(s)/pi(s)) every pd(s)/pi(s) must have an era commons account. pd(s)/pi(s) should work with their organizational officials to either create a new account or to affiliate their existing account with the applicant organization in era commons. if the pd/pi is also the organizational signing official, they must have two distinct era commons accounts, one for each role. obtaining an era commons account can take up to 2 weeks. all other personnel listed on the sf424 (r&r) senior/key person profile must have an era commons account. eligible individuals (program director/principal investigator) nih invites anyone who has the skills, knowledge, and resources needed to carry out the proposed research as a program director or principal investigator (pd/pi) to work through their organization to apply. individuals from diverse backgrounds, including underrepresented racial and ethnic groups, individuals with disabilities, and women are always encouraged to apply for nih support. see, reminder: notice of nih's encouragement of applications supporting individuals from underrepresented ethnic and racial groups as well as individuals with disabilities , not-od-22-019 . for institutions and organizations who are proposing multiple pds/pis, visit the senior/key person profile (expanded) component of the research instructions and look for submission details. eligibility is limited to individuals aiming to focus on aging research within their specialty/discipline who are: physicians (i.e., those with m.d., d.o., or equivalent degrees) who have completed training in their medical or surgical specialty. physician specialties include, but are not limited to, the traditional medical and surgical specialties, anesthesiology, emergency medicine, family medicine, general internal medicine, general surgery, geriatrics, hospital medicine, neurology, obstetrics/gynecology, palliative medicine, physiatry/rehabilitation medicine, and psychiatry; or dentists and dental specialists (i.e., those with either a d.d.s., d.m.d., or equivalent dental degree). this award is not intended for individuals with substantial experience in aging research. it is generally expected that applicants will not have had prior research funding as an independent pd/pi on a major nih grant (e.g., r01, p01), though they may have had support through institutional awards, such as institutional career development awards (e.g., k12, kl2) or nia center research education awards. note that nih policy now allows investigators with k awards to receive salary compensation from federal or non-federal sources for effort not directly supported by their k award (see not-od-17-094 ). candidates who have received individual mentored k-awards from nia (excluding k38 and k99) are generally ineligible. early-career clinician-scientists who have had a previous nih individual mentored career development award in a non-aging-related field and who wish to refocus their research efforts on aging-related science are eligible to apply for a gemsstar award. however, such individuals must provide strong justification in their application explaining how their proposed research will entail a meaningful shift towards aging-related science within their clinical specialty or discipline. such applicants are strongly encouraged to discuss their eligibility with nia program staff early in the application process. applicants considering applying for a mentored k award and a gemsstar award concurrently are strongly encouraged to contact nia program staff to ensure their plans are consistent with nih policy and nia programmatic interests. the aims of the two applications must not overlap per nih submission policies. nia will weigh an applicant's funding from other nia and nih awards in making gemsstar funding decisions. eligibility is limited to candidates who have, or will have, a faculty appointment at the start of gemsstar funding. candidates in the last year of training should provide documentation of institutional commitment for a faculty appointment as described in letters of support in section iv. faculty appointments that are contingent on receiving a gemsstar award are not acceptable. it is expected that gemsstar applications will have a single pd/pi and that other personnel will be listed as collaborators or consultants. answers to frequently asked questions regarding gemsstar eligibility can be found on nia's gemsstar webpage . 2. cost sharing.
This NOFO does not require cost sharing as defined in the NIH Grants Policy Statement NIH Grants Policy Statement Section 1.2 Definition of Terms.
3. Additional Information on Eligibility
Number of Applications
Applicant organizations may submit more than one application if each is scientifically distinct. NIH will not accept duplicate or highly overlapping applications under review at the same time. See the policy on Submission of Resubmission Application .
This means that NIH will not accept:
- A new (A0) application submitted before NIH issues the summary statement from the review of an overlapping new (A0) or resubmission (A1) application.
- A resubmission (A1) application submitted before NIH issues the summary statement from the review of the previous new (A0) application.
- An application that has substantial overlap with another application pending appeal of initial peer review. See the policy on Similar, Essentially Identical, or Identical Applications .
Section IV. Application and Submission Information
1. requesting an application package.
The application forms package specific to this opportunity must be accessed through ASSIST, Grants.gov Workspace or an institutional system-to-system solution. Links to apply using ASSIST or Grants.gov Workspace are available in Part 1 of this NOFO. See your administrative office for instructions if you plan to use an institutional system-to-system solution.
2. Content and Form of Application Submission
Applicants must follow the instructions in the Research (R) Instructions in the How to Apply - Application Guide unless this NOFO says otherwise. NIH strictly enforces these requirements. If the applicant does not follow them, NIH may delay or not accept the application for review. NIH encourages organizations to submit applications in advance of deadlines.
Letter of Intent
NIH asks that applicant organizations let us know if they plan to apply for this opportunity. The purpose is to plan for the number of expert reviewers needed to evaluate applications. It is not required.
By the date listed in Part 1. Overview Information , prospective applicants are asked to submit a letter of intent that includes the following information:
- Descriptive title of proposed activity
- The funding opportunity number and title
- Participating institution(s)
- The name(s), phone number, and email address of all the PD(s)/PI(s)
- Names of other key personnel
Please email the optional letter of intent to:
Ramesh Vemuri, Ph.D. National Institute on Aging (NIA) Telephone: 301-496-9666 Email: [email protected]
Page Limitations
All page limitations described in the How to Apply – Application Guide and the Table of Page Limits must be followed.
The following section supplements the instructions found in the How to Apply – Application Guide and should be used for preparing an application to this NOFO.
SF424(R&R) Cover
All instructions in the How to Apply - Application Guide must be followed.
SF424(R&R) Project/Performance Site Locations
Sf424(r&r) other project information.
Other attachments : Applicants must include an attachment titled "Professional Development Plan" that describes an individualized plan to garner resources, activities, collaborations, and/or didactic or practical experiences concurrent with the R03 research project that will 1) lead to enhanced knowledge and skills in aging/geriatrics science, and 2) increase the applicant's likelihood of successful completion of their proposed GEMSSTAR project. This plan would complement the GEMSSTAR R03 award and would need to be supported independent of the R03 research plan and funding.
This section is limited to 5 pages.
The elements of a Professional Development Plan may differ for each applicant based on career stage, research experience, institutional resources, or other factors. Applicants are encouraged to tailor their plans to meet their individual professional development needs in relation to their proposed GEMSSTAR project. Moreover, applicants are encouraged to describe specific activities that will augment their skills in geriatrics and/or aging research in order to develop into independent researchers in aging science.
Additional information on designing a Professional Development Plan, including answers to frequently asked questions, can be found on NIA's GEMSSTAR webpage .
SF424(R&R) Senior/Key Person Profile
Applicants should use the biographical sketch of the principal investigator to illustrate how this award will advance the investigator's career in aging research. Applicants should use biographical sketches of all investigators to illustrate complementary and integrated expertise in aging and/or geriatrics among the research team, and the appropriateness of each person's role to advance the specific aims of the project.
R&R or Modular Budget
Applicants should budget for travel to the annual GEMSSTAR grantees meeting supported by the Clin-STAR Coordinating Center . Except in unusual circumstances, only the PD/PI may be supported by R03 funds to travel to the grantees meeting. There is no specific line item in the Modular Budget format in which to list travel costs; instead, the proposed travel should be described in the budget justification section.
Answers to frequently asked questions regarding travel to the grantees meeting can be found on NIA's GEMSSTAR webpage .
R&R Subaward Budget
Phs 398 cover page supplement, phs 398 research plan.
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions:
Research Strategy: In accordance with the goals of the GEMSSTAR program, the Research Strategy should emphasize how the candidate's proposed research will advance the understanding of aging- or geriatrics-related science in the candidate’s specialty/discipline, as well as facilitate the candidate’s transition specifically into aging research. Applicants should address to what extent the proposed project introduces aging-related research questions to the applicant’s clinical specialty/disciplinary area, as well as its potential contribution to new and meaningful knowledge of geriatric or gerontologic science related to the applicant’s clinical specialty/discipline.
Clinical Trials: Without duplicating information in the PHS Human Subjects and Clinical Trials Information Form, applicants proposing a clinical trial, ancillary, or feasibility study should describe the planned analyses and statistical approach and how the expected analytical approach is suited to the available resources, proposed study design, project scope, and methods used to assign trial participants and deliver interventions.
If proposing an ancillary study to an ongoing clinical trial, applicants should provide a brief description of its relationship to the larger clinical trial.
If proposing a feasibility study to begin to address a clinical question, applicants should provide justification as to why this is warranted and how it will contribute to the overall goals of the research project, including planning and preliminary data for future, larger-scale clinical trials.
Applicants should describe the proposed timelines for the proposed clinical trial, feasibility, or ancillary study, including any potential challenges and solutions (e.g., enrollment shortfalls or inability to attribute causal inference to the results of an intervention when performing a small feasibility study).
Letters of Support: Letters of support from department or division chairs, other institutional leaders, senior collaborators, and other contributors should describe, where appropriate, the following:
- Support from the applicant's institution, department, or division for the applicant's successful completion of the proposed research project
- Efforts from the letter's author to promote transdisciplinary aging research within the author's specialty/discipline
- Scientific roles of senior collaborator(s) or other contributors to the applicant's professional development
- For applicants in their last year of training, documentation of the institution's commitment to the applicant's faculty appointment, which should commence no later than the start of the GEMSSTAR R03 award
Letters of support associated with a Professional Development Plan, if included, should be placed in the Letters of Support section. Applicants are encouraged to visit NIA's GEMSSTAR webpage for additional information regarding letters of support.
Resource Sharing Plan : Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the How to Apply - Application Guide .
Other Plan(s):
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions:
- All applicants planning research (funded or conducted in whole or in part by NIH) that results in the generation of scientific data are required to comply with the instructions for the Data Management and Sharing Plan. All applications, regardless of the amount of direct costs requested for any one year, must address a Data Management and Sharing Plan.
Appendix: Only limited Appendix materials are allowed. Follow all instructions for the Appendix as described in the How to Apply - Application Guide .
- No publications or other material, with the exception of blank questionnaires or blank surveys, may be included in the Appendix.
PHS Human Subjects and Clinical Trials Information
When involving human subjects research, clinical research, and/or NIH-defined clinical trials (and when applicable, clinical trials research experience) follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the How to Apply - Application Guide , with the following additional instructions:
If the answer to the question “Are Human Subjects Involved?” on the R&R Other Project Information form is yes, include at least one human subjects study record. Use either of the following parts of the PHS Human Subjects and Clinical Trials Information form:
- Study Record
Delayed Onset Study
Study Record: PHS Human Subjects and Clinical Trials Information
Section 2 - Study Population Characteristics
2.2 Eligibility Criteria
Applications in response to this funding opportunity must provide a rationale supporting eligibility criteria that are 1) representative of the population affected by the disease/condition, and 2) address the populations outlined in the NIH Inclusion Policies for Research Involving Human Subjects and NIH-designated Populations with Health Disparities , as appropriate ; as well as other populations that experience health disparities. The goal is for clinical trials to address inclusion, so that researchers can determine whether the variables being studied affect women or members of any racial and ethnic population group in accordance with the NIH Inclusion Policies.
Study teams must demonstrate that they have considered the NIH Inclusion Policies including proposed planned enrollment tables representative of the population affected by the disease/condition. Where applicable, study teams should also demonstrate that they have critically evaluated whether eligibility criteria from an earlier phase trial should be carried forward into a later phase trial. The eligibility criteria section should:
- Describe how the study results generalize to the wider patient population with this disease/condition.
- Explain how the rationale for selected eligibility criteria justify the level of restriction in the study compared to clinical practice.
- Provide evidence that the eligibility criteria support the proposed research and encourage inclusion of N IH-designated Populations with Health Disparities .
2.5 Recruitment and Retention Plan
Applications in response to this funding opportunity should propose innovative and proactive recruitment strategies for involving understudied populations to promote representation as applicable and justified by the scientific goals. Applicants should ensure that they 1) include proposed planned enrollment tables identifying the population(s) affected by the disease/condition, and 2) address the populations outlined in the NIH Inclusion Policies for Research Involving Human Subjects and NIH-designated Populations with Health Disparities , as appropriate ; as well as other populations that experience health disparities. Recruitment and retention plans should demonstrate an understanding of the participant burden involved in research participation and strategies for minimizing this burden, as well as leveraging community partners and outreach efforts. The recruitment and retention plan should:
- Describe potential barriers to participation and plans to minimize these barriers.
- Describe a detailed plan for the recruitment of understudied populations and a plan to leverage existing relationships with and/or conduct outreach to a broad range of community groups.
- Describe staff training to address cultural and linguistic competence .
Note: Delayed onset is not the same as a delayed start study that can be described but will not start immediately.
PHS Assignment Request Form
3. unique entity identifier and system for award management (sam).
See Part 2. Section III.1 for information regarding the requirement for obtaining a unique entity identifier and for completing and maintaining active registrations in System for Award Management (SAM), NATO Commercial and Government Entity (NCAGE) Code (if applicable), eRA Commons, and Grants.gov
4. Submission Dates and Times
Part I. contains information about Key Dates and times. Applicants should apply early to allow time to make corrections to errors found in the application during the submission process before the due date. When a due date falls on a weekend or Federal holiday , the application deadline is automatically extended to the next business day.
Organizations must submit applications to Grants.gov (the online portal to find and apply for grants across all Federal agencies). Applicants must then complete the submission process by tracking the status of the application in the eRA Commons , NIH’s electronic system for grants administration. NIH and Grants.gov systems check the application against many of the application instructions upon submission. Errors must be corrected and a changed/corrected application must be submitted to Grants.gov on or before the application due date and time. If a Changed/Corrected application is submitted after the deadline, the application will be considered late. Applications that miss the due date and time are subjected to the NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications .
Applicants are responsible for viewing their application before the due date in the eRA Commons to ensure accurate and successful submission.
Information on the submission process and a definition of on-time submission are provided in the How to Apply – Application Guide .
5. Intergovernmental Review (E.O. 12372)
This initiative is not subject to intergovernmental review.
6. Funding Restrictions
- All NIH awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
- NIH allows pre-award costs only as described in the NIH Grants Policy Statement Section 7.9.1 Selected Items of Cost .
7. Other Submission Requirements and Information
Applications must be submitted electronically following the instructions described in the How to Apply - Application Guide . Paper applications will not be accepted.
Applicants must complete all required registrations before the application due date. Section III. Eligibility Information contains information about registration.
For assistance with your electronic application or for more information on the electronic submission process, visit How to Apply – Application Guide . F ollow the overall instructions at How to Submit, Track and View Your Application on our website.
If you encounter a system issue beyond your control that impacts the ability to complete the submission process on-time, follow the Dealing with System Issues guidance. For assistance with application submission, contact the Application Submission Contacts in Section VII.
Important reminders:
All PD(s)/PI(s) must have an eRA Commons ID account that is affiliated with the applicant organization in eRA Commons.
If the PD/PI is also the organizational signing official, they must have two distinct eRA Commons accounts, one for each role.
All other personnel listed on the SF424 (R&R) Senior/Key Person Profile must have an eRA Commons account.
See Section III of this NOFO for information on registration requirements.
The applicant organization must ensure that the unique entity identifier provided on the application is the same identifier used in the organization’s profile in the eRA Commons and for the System for Award Management. Additional information may be found in the How to Apply - Application Guide .
See more tips for avoiding common errors.
Upon receipt, applications will be evaluated for completeness and compliance with application instructions by the Center for Scientific Review and responsiveness by NIA , NIH. Applications that are incomplete, non-compliant, and/or nonresponsive will not be reviewed.
Recipients or subrecipients must submit any information related to violations of federal criminal law involving fraud, bribery, or gratuity violations potentially affecting the federal award. See Mandatory Disclosures, 2 CFR 200.113 and NIH Grants Policy Statement Section 4.1.35 .
Send written disclosures to the NIH Chief Grants Management Officer listed on the Notice of Award for the IC that funded the award and to the HHS Office of Inspector Grant Self Disclosure Program at [email protected] .
Post Submission Materials
Applicants are required to follow the instructions for post-submission materials, as described in the policy
Section V. Application Review Information
1. criteria.
Only the review criteria described below will be considered in the review process. Applications submitted to the NIH in support of the NIH mission are evaluated for scientific and technical merit through the NIH peer review system.
For this particular NOFO, note the following:
The R03 small grant supports discrete, well-defined projects that realistically can be completed in two years and that require limited levels of funding. Because the research project usually is limited, an R03 grant application may not contain extensive detail or discussion. Accordingly, reviewers should evaluate the conceptual framework and general approach to the problem. Appropriate justification for the proposed work can be provided through literature citations, data from other sources, or from investigator-generated data. Preliminary data are not required, particularly in applications proposing pilot or feasibility studies.
A proposed Clinical Trial application may include study design, methods, and intervention that are not by themselves innovative but address important questions or unmet needs. Additionally, the results of the clinical trial may indicate that further clinical development of the intervention is unwarranted or lead to new avenues of scientific investigation.
Reviewers will provide an overall impact score to reflect their assessment of the likelihood for the project to exert a sustained, powerful influence on the research field(s) involved, in consideration of the following review criteria and additional review criteria (as applicable for the project proposed).
Reviewers will consider each of the review criteria below in the determination of scientific merit and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact. For example, a project that by its nature is not innovative may be essential to advance a field.
Does the project address an important problem or a critical barrier to progress in the field? Is the prior research that serves as the key support for the proposed project rigorous? If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field?
In addition, for applications involving clinical trials
Are the scientific rationale and need for a clinical trial to test the proposed hypothesis or intervention well supported by preliminary data, clinical and/or preclinical studies, or information in the literature or knowledge of biological mechanisms? For trials focusing on clinical or public health endpoints, is this clinical trial necessary for testing the safety, efficacy or effectiveness of an intervention that could lead to a change in clinical practice, community behaviors or health care policy? For trials focusing on mechanistic, behavioral, physiological, biochemical, or other biomedical endpoints, is this trial needed to advance scientific understanding?
Are the PD(s)/PI(s), collaborators, and other researchers well suited to the project? If Early Stage Investigators or those in the early stages of independent careers, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If the project is collaborative or multi-PD/PI, do the investigators have complementary and integrated expertise; are their leadership approach, governance and organizational structure appropriate for the project?
With regard to the proposed leadership for the project, do the PD/PI(s) and key personnel have the expertise, experience, and ability to organize, manage and implement the proposed clinical trial and meet milestones and timelines? Do they have appropriate expertise in study coordination, data management and statistics? For a multicenter trial, is the organizational structure appropriate and does the application identify a core of potential center investigators and staffing for a coordinating center?
Specific to this NOFO:
To what extent does the Professional Development Plan promote the candidate’s knowledge and skills in geriatrics- or aging-related research?
Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation, or interventions? Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense? Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, instrumentation, or interventions proposed?
Does the design/research plan include innovative elements, as appropriate, that enhance its sensitivity, potential for information or potential to advance scientific knowledge or clinical practice?
Are the overall strategy, methodology, and analyses well-reasoned and appropriate to accomplish the specific aims of the project? Have the investigators included plans to address weaknesses in the rigor of prior research that serves as the key support for the proposed project? Have the investigators presented strategies to ensure a robust and unbiased approach, as appropriate for the work proposed? Are potential problems, alternative strategies, and benchmarks for success presented? If the project is in the early stages of development, will the strategy establish feasibility and will particularly risky aspects be managed? Have the investigators presented adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects?
If the project involves human subjects and/or NIH-defined clinical research, are the plans to address 1) the protection of human subjects from research risks, and 2) inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion or exclusion of individuals of all ages (including children and older adults), justified in terms of the scientific goals and research strategy proposed?
Does the application adequately address the following, if applicable
Study Design
Is the study design justified and appropriate to address primary and secondary outcome variable(s)/endpoints that will be clear, informative and relevant to the hypothesis being tested? Is the scientific rationale/premise of the study based on previously well-designed preclinical and/or clinical research? Given the methods used to assign participants and deliver interventions, is the study design adequately powered to answer the research question(s), test the proposed hypothesis/hypotheses, and provide interpretable results? Is the trial appropriately designed to conduct the research efficiently? Are the study populations (size, gender, age, demographic group), proposed intervention arms/dose, and duration of the trial, appropriate and well justified?
Are potential ethical issues adequately addressed? Is the process for obtaining informed consent or assent appropriate? Is the eligible population available? Are the plans for recruitment outreach, enrollment, retention, handling dropouts, missed visits, and losses to follow-up appropriate to ensure robust data collection? Are the planned recruitment timelines feasible and is the plan to monitor accrual adequate? Has the need for randomization (or not), masking (if appropriate), controls, and inclusion/exclusion criteria been addressed? Are differences addressed, if applicable, in the intervention effect due to sex/gender and race/ethnicity?
Are the plans to standardize, assure quality of, and monitor adherence to, the trial protocol and data collection or distribution guidelines appropriate? Is there a plan to obtain required study agent(s)? Does the application propose to use existing available resources, as applicable?
Data Management and Statistical Analysis
Are planned analyses and statistical approach appropriate for the proposed study design and methods used to assign participants and deliver interventions? Are the procedures for data management and quality control of data adequate at clinical site(s) or at center laboratories, as applicable? Have the methods for standardization of procedures for data management to assess the effect of the intervention and quality control been addressed? Is there a plan to complete data analysis within the proposed period of the award?
Eligibility Criteria for Research Involving Human Subjects
To what extent do the eligibility criteria promote inclusion of the population affected by the disease/condition? To what extent is justification provided for eligibility criteria, including inclusion and exclusion of NIH-designated Populations with Health Disparities ? Have barriers to participation been assessed adequately?
Recruitment and Retention Plan for Research Involving Human Subjects
How well does the recruitment and retention plan demonstrate efforts to engage understudied populations in the clinical trial, as applicable and justified by the scientific goals? To what extent will the recruitment efforts increase community engagement, reduce identified barriers, and sustain the engagement of understudied populations? To what extent are plans described to train staff to be sensitive to NIH-designated Populations with Health Disparities ?
Will the scientific environment in which the work will be done contribute to the probability of success? Are the institutional support, equipment and other physical resources available to the investigators adequate for the project proposed? Will the project benefit from unique features of the scientific environment, subject populations, or collaborative arrangements?
If proposed, are the administrative, data coordinating, enrollment and laboratory/testing centers, appropriate for the trial proposed?
Does the application adequately address the capability and ability to conduct the trial at the proposed site(s) or centers? Are the plans to add or drop enrollment centers, as needed, appropriate?
If international site(s) is/are proposed, does the application adequately address the complexity of executing the clinical trial?
If multi-sites/centers, is there evidence of the ability of the individual site or center to: (1) enroll the proposed numbers; (2) adhere to the protocol; (3) collect and transmit data in an accurate and timely fashion; and, (4) operate within the proposed organizational structure?
To what extent does the Professional Development Plan reflect a supportive environment that will facilitate promoting the candidate’s knowledge and skills in geriatrics- or aging-related research?
As applicable for the project proposed, reviewers will evaluate the following additional items while determining scientific and technical merit, and in providing an overall impact score, but will not give separate scores for these items.
Specific to applications involving clinical trials
Is the study timeline described in detail, taking into account start-up activities, the anticipated rate of enrollment, and planned follow-up assessment? Is the projected timeline feasible and well justified? Does the project incorporate efficiencies and utilize existing resources (e.g., CTSAs, practice-based research networks, electronic medical records, administrative database, or patient registries) to increase the efficiency of participant enrollment and data collection, as appropriate?
Are potential challenges and corresponding solutions discussed (e.g., strategies that can be implemented in the event of enrollment shortfalls)?
For research that involves human subjects but does not involve one of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate the justification for involvement of human subjects and the proposed protections from research risk relating to their participation according to the following five review criteria: 1) risk to subjects, 2) adequacy of protection against risks, 3) potential benefits to the subjects and others, 4) importance of the knowledge to be gained, and 5) data and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or more of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials. For additional information on review of the Human Subjects section, please refer to the Guidelines for the Review of Human Subjects .
When the proposed project involves human subjects and/or NIH-defined clinical research, the committee will evaluate the proposed plans for the inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion (or exclusion) of individuals of all ages (including children and older adults) to determine if it is justified in terms of the scientific goals and research strategy proposed. For additional information on review of the Inclusion section, please refer to the Guidelines for the Review of Inclusion in Clinical Research .
The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following three points: (1) a complete description of all proposed procedures including the species, strains, ages, sex, and total numbers of animals to be used; (2) justifications that the species is appropriate for the proposed research and why the research goals cannot be accomplished using an alternative non-animal model; and (3) interventions including analgesia, anesthesia, sedation, palliative care, and humane endpoints that will be used to limit any unavoidable discomfort, distress, pain and injury in the conduct of scientifically valuable research. Methods of euthanasia and justification for selected methods, if NOT consistent with the AVMA Guidelines for the Euthanasia of Animals, is also required but is found in a separate section of the application. For additional information on review of the Vertebrate Animals Section, please refer to the Worksheet for Review of the Vertebrate Animals Section.
Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
For Resubmissions, the committee will evaluate the application as now presented, taking into consideration the responses to comments from the previous scientific review group and changes made to the project.
Not Applicable.
As applicable for the project proposed, reviewers will consider each of the following items, but will not give scores for these items, and should not consider them in providing an overall impact score.
Reviewers will assess whether the project presents special opportunities for furthering research programs through the use of unusual talent, resources, populations, or environmental conditions that exist in other countries and either are not readily available in the United States or augment existing U.S. resources.
Reviewers will assess the information provided in this section of the application, including 1) the Select Agent(s) to be used in the proposed research, 2) the registration status of all entities where Select Agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of Select Agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the Select Agent(s).
Reviewers will comment on whether the Resource Sharing Plan(s) (e.g., Sharing Model Organisms ) or the rationale for not sharing the resources, is reasonable.
For projects involving key biological and/or chemical resources, reviewers will comment on the brief plans proposed for identifying and ensuring the validity of those resources.
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
2. Review and Selection Process Applications will be evaluated for scientific and technical merit by (an) appropriate Scientific Review Group(s) convened by NIA, in accordance with NIH peer review policy and procedures , using the stated review criteria . Assignment to a Scientific Review Group will be shown in the eRA Commons. As part of the scientific peer review, all applications will receive a written critique. Applications may undergo a selection process in which only those applications deemed to have the highest scientific and technical merit (generally the top half of applications under review) will be discussed and assigned an overall impact score. Appeals of initial peer review will not be accepted for applications submitted in response to this NOFO. Applications will be assigned to the appropriate NIH Institute or Center. Applications will compete for available funds with all other recommended applications submitted in response to this NOFO. Following initial peer review, recommended applications will receive a second level of review by the National Advisory Council on Aging. The following will be considered in making funding decisions: Scientific and technical merit of the proposed project as determined by scientific peer review. Availability of funds. Relevance of the proposed project to program priorities. This includes the following: The degree to which the proposed project represents a meaningful transition to a career devoted to aging-related research, or a significant first independent research effort, as opposed to an additive research experience. The extent to which a candidate's environment is supportive of aging- or geriatric-focused research If the application is under consideration for funding, NIH will request "just-in-time" information from the applicant as described in the NIH Grants Policy Statement Section 2.5.1. Just-in-Time Procedures . This request is not a Notice of Award nor should it be construed to be an indicator of possible funding. Before making an award, NIH conducts a pre-award risk assessment. The purpose is to make sure the applicant organization has handled any past federal awards well and demonstrated sound business practices. NIH uses SAM.gov Exclusions and Responsibility / Qualification to check this history for all awards likely to be over $250K.This provision will apply to all NIH grants and cooperative agreements except fellowships. The applicant organization can comment on the information in SAM.gov. NIH will consider the comments before making a decision about level of risk. If there is a significant risk, NIH may choose not to fund the application or to place specific conditions on the award. For more details, see 2 CFR Part 200.206. 3. Anticipated Announcement and Award Dates
After the peer review of the application is completed, the PD/PI will be able to access their Summary Statement (written critique) via the eRA Commons . Refer to Part 1 for dates for peer review, advisory council review, and earliest start date.
Information regarding the disposition of applications is available in the NIH Grants Policy Statement Section 2.4.4 Disposition of Applications .
Section VI. Award Administration Information
1. award notices.
If an application is successful, NIH will email a Notice of Award (NoA) to the authorized official. NIH will inform the applicant organization if their application is disqualified or unsuccessful.
The NoA is the only official award document. The NoA explains the amount of the award, important dates, and the terms and conditions recipients need to follow. Until the organization receives the NoA, the recipient does not have permission to begin the research project.
All awards are subject to terms and conditions at Award Conditions and Information for NIH Grants website.
Recipients must comply with any funding restrictions described in Section IV.6. Funding Restrictions . Any pre-award costs incurred before receipt of the NoA are at the applicant's own risk. For more information on the Notice of Award, please refer to the NIH Grants Policy Statement Section 5. The Notice of Award and NIH Grants & Funding website, see Award Process.
Individual awards are based on the application submitted to, and as approved by, the NIH and are subject to the IC-specific terms and conditions identified in the NoA.
ClinicalTrials.gov: If an award provides for one or more clinical trials. By law (Title VIII, Section 801 of Public Law 110-85), the "responsible party" must register and submit results information for certain “applicable clinical trials” on the ClinicalTrials.gov Protocol Registration and Results System Information Website ( https://register.clinicaltrials.gov ). NIH expects registration and results reporting of all trials whether required under the law or not. For more information, see https://grants.nih.gov/policy/clinical-trials/reporting/index.htm
Institutional Review Board or Independent Ethics Committee Approval: Recipient institutions must ensure that all protocols are reviewed by their IRB or IEC. To help ensure the safety of participants enrolled in NIH-funded studies, the recipient must provide NIH copies of documents related to all major changes in the status of ongoing protocols.
Data and Safety Monitoring Requirements: The NIH policy for data and safety monitoring requires oversight and monitoring of all NIH-conducted or -supported human biomedical and behavioral intervention studies (clinical trials) to ensure the safety of participants and the validity and integrity of the data. Further information concerning these requirements is found at http://grants.nih.gov/grants/policy/hs/data_safety.htm and in the application instructions (SF424 (R&R) and PHS 398).
Investigational New Drug or Investigational Device Exemption Requirements: Consistent with federal regulations, clinical research projects involving the use of investigational therapeutics, vaccines, or other medical interventions (including licensed products and devices for a purpose other than that for which they were licensed) in humans under a research protocol must be performed under a Food and Drug Administration (FDA) investigational new drug (IND) or investigational device exemption (IDE).
2. Administrative and National Policy Requirements
All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the terms and conditions in the Notice of Award (NoA) The NoA includes the requirements of this NOFO. For these terms, note the following:
- The rules listed at 2 CFR Part 200 , Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards.
- All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the terms and conditions in the Notice of Award (NoA). The NoA includes the requirements of this NOFO. For these terms of award, see the NIH Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards, Subpart A: General and Part II: Terms and Conditions of NIH Grant Awards, Subpart B: Terms and Conditions for Specific Types of Grants, Recipients, and Activities .
- HHS recognizes that NIH research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator’s scientific interest, funding limitations, recruitment requirements, and other considerations. Thus, criteria in research protocols that target or exclude certain populations are warranted where nondiscriminatory justifications establish that such criteria are appropriate with respect to the health or safety of the subjects, the scientific study design, or the purpose of the research. For additional guidance regarding how the provisions apply to NIH grant programs, please contact the Scientific/Research Contact that is identified in Section VII under Agency Contacts of this NOFO.
All federal statutes and regulations relevant to federal financial assistance, including those highlighted in NIH Grants Policy Statement Section 4 Public Policy Requirements, Objectives and Other Appropriation Mandates.
Recipients are responsible for ensuring that their activities comply with all applicable federal regulations. NIH may terminate awards under certain circumstances. See 2 CFR Part 200.340 Termination and NIH Grants Policy Statement Section 8.5.2 Remedies for Noncompliance or Enforcement Actions: Suspension, Termination, and Withholding of Support .
3. Data Management and Sharing
Consistent with the 2023 NIH Policy for Data Management and Sharing, when data management and sharing is applicable to the award, recipients will be required to adhere to the Data Management and Sharing requirements as outlined in the NIH Grants Policy Statement . Upon the approval of a Data Management and Sharing Plan, it is required for recipients to implement the plan as described.
4. Reporting
When multiple years are involved, recipients will be required to submit the Research Performance Progress Report (RPPR) annually and financial statements as required in the NIH Grants Policy Statement Section 8.4.1 Reporting. To learn more about post-award monitoring and reporting, see the NIH Grants & Funding website, see Post-Award Monitoring and Reporting .
A final RPPR, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the NIH Grants Policy Statement Section 8.6 Closeout . NIH NOFOs outline intended research goals and objectives. Post award, NIH will review and measure performance based on the details and outcomes that are shared within the RPPR, as described at 2 CFR Part 200.301.
Section VII. Agency Contacts
We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants.
eRA Service Desk
Contact the eRA Service Desk for questions about ASSIST, eRA Commons, application errors and warnings, documenting system problems that threaten submission by the due date, and post-submission issues.
Finding Help Online: https://www.era.nih.gov/need-help (preferred method of contact) Telephone: 301-402-7469 or 866-504-9552 (Toll Free)
General Grants Information
Contact Grants Information for questions about application instructions, application processes, and NIH grant resources. Email: [email protected] (preferred method of contact) Telephone: 301-480-7075
Grants.gov Customer Support
Contact Grants.gov Customer Support for questions about Grants.gov registration and Workspace. Contact Center Telephone: 800-518-4726 Email: [email protected]
NIA GEMSSTAR National Institute on Aging (NIA) Email: [email protected]
Laura Pone National Institute on Aging (NIA) Telephone: 301-451-9956 Email: [email protected]
Section VIII. Other Information
Recently issued trans-NIH policy notices may affect your application submission. A full list of policy notices published by NIH is provided in the NIH Guide for Grants and Contracts . All awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
NIH makes awards under the authorization of Sections 301 and 405 of the Public Health Service Act as amended (42 USC 241 and 284) and under Federal Regulations 42 CFR Part 52 and 2 CFR Part 200.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The clinician's guide to prevention and treatment of osteoporosis
Affiliations.
- 1 Brigham and Women's Hospital, Harvard Medical School, 221 Longwood Ave, Boston, MA, 02115, USA. [email protected].
- 2 University of Pittsburgh Medical Center, 1110 Kaufmann Building, 3471 Fifth Ave, Pittsburgh, PA, 15213, USA.
- 3 Yale School of Medicine, 333 Cedar St, New Haven, CT, 06520, USA.
- 4 University of New Mexico Health Sciences Center, 300 Oak St NE, Albuquerque, NM, 87106, USA.
- 5 University of Alabama at Birmingham, 1720 2nd Avenue South, FOT 820, Birmingham, AL, 35294, USA.
- 6 MedStar Georgetown University Hospital and Georgetown University Medical Center, 3800 Reservoir Road NW, 3rd Floor, Washington, DC, 20007, USA.
- 7 Columbia University Irving Medical Center, 180 Fort Washington Ave, Suite 9-903, New York, NY, 10032, USA.
- PMID: 35478046
- PMCID: PMC9546973
- DOI: 10.1007/s00198-021-05900-y
- Correction to: The clinician's guide to prevention and treatment of osteoporosis. LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, Siris ES. LeBoff MS, et al. Osteoporos Int. 2022 Oct;33(10):2243. doi: 10.1007/s00198-022-06479-8. Osteoporos Int. 2022. PMID: 35900384 Free PMC article. No abstract available.
Osteoporosis is the most common metabolic bone disease in the USA and the world. It is a subclinical condition until complicated by fracture(s). These fractures place an enormous medical and personal burden on individuals who suffer from them and take a significant economic toll. Any new fracture in an adult aged 50 years or older signifies imminent elevated risk for subsequent fractures, particularly in the year following the initial fracture. What a patient perceives as an unfortunate accident may be seen as a sentinel event indicative of bone fragility and increased future fracture risk even when the result of considerable trauma. Clinical or subclinical vertebral fractures, the most common type of osteoporotic fractures, are associated with a 5-fold increased risk for additional vertebral fractures and a 2- to 3-fold increased risk for fractures at other sites. Untreated osteoporosis can lead to a vicious cycle of recurrent fracture(s), often resulting in disability and premature death. In appropriate patients, treatment with effective antifracture medication prevents fractures and improves outcomes. Primary care providers and medical specialists are critical gatekeepers who can identify fractures and initiate proven osteoporosis interventions. Osteoporosis detection, diagnosis, and treatment should be routine practice in all adult healthcare settings. The Bone Health and Osteoporosis Foundation (BHOF) - formerly the National Osteoporosis Foundation - first published the Clinician's Guide in 1999 to provide accurate information on osteoporosis prevention and treatment. Since that time, significant improvements have been made in diagnostic technologies and treatments for osteoporosis. Despite these advances, a disturbing gap persists in patient care. At-risk patients are often not screened to establish fracture probability and not educated about fracture prevention. Most concerning, the majority of highest risk women and men who have a fracture(s) are not diagnosed and do not receive effective, FDA-approved therapies. Even those prescribed appropriate therapy are unlikely to take the medication as prescribed. The Clinician's Guide offers concise recommendations regarding prevention, risk assessment, diagnosis, and treatment of osteoporosis in postmenopausal women and men aged 50 years and older. It includes indications for bone densitometry as well as fracture risk thresholds for pharmacologic intervention. Current medications build bone and/or decrease bone breakdown and dramatically reduce incident fractures. All antifracture therapeutics treat but do not cure the disease. Skeletal deterioration resumes sooner or later when a medication is discontinued-sooner for nonbisphosphonates and later for bisphosphonates. Even if normal BMD is achieved, osteoporosis and elevated risk for fracture are still present. The diagnosis of osteoporosis persists even if subsequent DXA T-scores are above - 2.5. Ongoing monitoring and strategic interventions will be necessary if fractures are to be avoided. In addition to pharmacotherapy, adequate intake of calcium and vitamin D, avoidance of smoking and excessive alcohol intake, weight-bearing and resistance-training exercise, and fall prevention are included in the fracture prevention armamentarium. Where possible, recommendations in this guide are based on evidence from RCTs; however, relevant published data and guidance from expert clinical experience provides the basis for recommendations in those areas where RCT evidence is currently deficient or not applicable to the many osteoporosis patients not considered for RCT participation due to age and morbidity.
Keywords: Bisphosphonate holiday; FRAX®; Fracture risk stratification; Fractures; Novel antifracture therapies (romosozumab, denosumab, abaloparatide); Osteoporosis; Primary care management of osteoporosis; Vertebral imaging.
© 2022. The Author(s).
PubMed Disclaimer
Conflict of interest statement
2020 Clinician’s Guide Update Committee: Meryl S. LeBoff, MD; NIA R01 AG071611; NIAMS R01 AR070854; NIAMS R01 AR059775; Amgen; Susan L. Greenspan, MD, no disclosures; Karl Insogna, MD, no disclosures; E. Michael Lewiecki, MD, Radius, Amgen, Mereo, Bindex, Alexion; Kenneth G. Saag, MD, no disclosures; Andrea Singer, MD, Amgen, Radius Health, UCB; Ethel S. Siris, MD, no disclosures. Subject Specialist Contributors: Kathryn E. Ackerman, MD, MPH, FACSM; Douglas C Bauer, MD, no disclosures; Theresa Chiaia PT, DPT no disclosures; Polly de Mille RN, MA, RCEP, CSCS, USAT, no disclosures; Thomas F. Koinis, MD, no disclosures; Wendy Katzman, PT, DPTSc (DSc), OCS, no disclosures; Rick Pope MPAS, PA-C, DFAAPA, CPAAPA, no disclosures; Heidi Skolnik, MS, CDN, FACSM, American Dairy Association, Sport Advisory Panel. Bone Health and Osteoporosis Foundation Staff: Claire Gill no disclosures, Ami R. Patel no disclosures, Kelly A. Trippe no disclosures.
Hip fracture incidence in postmenopausal…
Hip fracture incidence in postmenopausal women across ethic/racial populations in WHI data (from…
Incidence of hip fractures (age-adjusted)…
Incidence of hip fractures (age-adjusted) between 2002 and 2015 according to Medicare claims.…
Micrographs of normal (left) and…
Micrographs of normal (left) and osteoporotic (right) bone. As trabecular mineral is depleted,…
Hip BMD showing low bone…
Hip BMD showing low bone mass and a history of a fracture. The…
This contrast between percentage of…
This contrast between percentage of people in general population who use wheelchairs (0.859…
Management of long-term bisphosphonate (BP)…
Management of long-term bisphosphonate (BP) treatment in postmenopausal women. Note: This flowchart illustrates…
Daily activities and household chores…
Daily activities and household chores can be modified to minimize risk for vertebral…
For people with osteoporosis, the…
For people with osteoporosis, the harm or benefit conferred by exercise depends on…
Similar articles
- Summary of AHRQ's comparative effectiveness review of treatment to prevent fractures in men and women with low bone density or osteoporosis: update of the 2007 report. Levis S, Theodore G. Levis S, et al. J Manag Care Pharm. 2012 May;18(4 Suppl B):S1-15; discussion S13. doi: 10.18553/jmcp.2012.18.s4-b.1. J Manag Care Pharm. 2012. PMID: 22716221 Free PMC article.
- Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation. Davis S, Simpson E, Hamilton J, James MM, Rawdin A, Wong R, Goka E, Gittoes N, Selby P. Davis S, et al. Health Technol Assess. 2020 Jun;24(29):1-314. doi: 10.3310/hta24290. Health Technol Assess. 2020. PMID: 32588816 Free PMC article.
- Utilization of DXA Bone Mineral Densitometry in Ontario: An Evidence-Based Analysis. Medical Advisory Secretariat. Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2006;6(20):1-180. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074491 Free PMC article.
- Primary care use of FRAX: absolute fracture risk assessment in postmenopausal women and older men. Siris ES, Baim S, Nattiv A. Siris ES, et al. Postgrad Med. 2010 Jan;122(1):82-90. doi: 10.3810/pgm.2010.01.2102. Postgrad Med. 2010. PMID: 20107292 Review.
- Managing osteoporosis in ulcerative colitis: something new? Piodi LP, Poloni A, Ulivieri FM. Piodi LP, et al. World J Gastroenterol. 2014 Oct 21;20(39):14087-98. doi: 10.3748/wjg.v20.i39.14087. World J Gastroenterol. 2014. PMID: 25339798 Free PMC article. Review.
- Real-world efficacy of a teriparatide biosimilar (RGB-10) compared with reference teriparatide on bone mineral density, trabecular bone score, and bone parameters assessed using quantitative ultrasound, 3D-SHAPER ® and high-resolution peripheral computer tomography in postmenopausal women with osteoporosis and very high fracture risk. Hadji P, Kamali L, Thomasius F, Horas K, Kurth A, Bock N. Hadji P, et al. Osteoporos Int. 2024 Aug 2. doi: 10.1007/s00198-024-07208-z. Online ahead of print. Osteoporos Int. 2024. PMID: 39093439
- An Outline on the Advancements in Surgical Management of Osteoporosis-Associated Fractures. Hakami IA. Hakami IA. Cureus. 2024 Jun 26;16(6):e63226. doi: 10.7759/cureus.63226. eCollection 2024 Jun. Cureus. 2024. PMID: 39070522 Free PMC article. Review.
- Importance of Bilateral Hip Assessments in Unilateral Lower-Limb Amputees: A Retrospective Review Involving Older Veterans. Jin S, An CH, Jeong HY, Choi W, Hong SW, Song HK, Kim HS, Lee YK, Kang HJ, Ahn DY, Yang HE. Jin S, et al. J Clin Med. 2024 Jul 10;13(14):4033. doi: 10.3390/jcm13144033. J Clin Med. 2024. PMID: 39064073 Free PMC article.
- The Effects of Acute and Chronic Alcohol Administration and Withdrawal on Bone Microstructure, Mechanical Strength, and Remodeling Protein Expression and Their Relation to an Antioxidant and FGF23 In Vivo. Syed Hashim SA, Naina Mohamed I, Mohamed N. Syed Hashim SA, et al. Biomedicines. 2024 Jul 8;12(7):1515. doi: 10.3390/biomedicines12071515. Biomedicines. 2024. PMID: 39062088 Free PMC article.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Endocrine Society et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. - DOI - PubMed
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. - DOI - PMC - PubMed
- Dudenkov DV, Yawn BP, Oberhelman SS, et al. Changing incidence of serum 25-hydroxyvitamin d values above 50 ng/mL: a 10-year population-based study. Mayo Clin Proc. 2015;90(5):577–586. doi: 10.1016/j.mayocp.2015.02.012. - DOI - PMC - PubMed
- Leslie WD, Schousboe JT, Morin SN, et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int Jun. 2020;31(6):1059–1067. doi: 10.1007/s00198-019-05274-2. - DOI - PMC - PubMed
- Mackey DC, Lui LY, Cawthon PM, Study of Osteoporotic Fractures (SOF) and Osteoporotic Fractures in Men Study (MrOS) Research Groups et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298(20):2381–2388. doi: 10.1001/jama.298.20.2381. - DOI - PubMed
- Search in MeSH
Related information
- Cited in Books
- PubChem Compound (MeSH Keyword)
Grants and funding
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 AR059775/AR/NIAMS NIH HHS/United States
- R01 AG071611/AG/NIA NIH HHS/United States
- R01 AR070854/AR/NIAMS NIH HHS/United States
- R01 AR060574/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- PubMed Central
- Genetic Alliance
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Research Funded by NIMH
- Research Conducted at NIMH (Intramural Research Program)
- Priority Research Areas
- Research Resources
Ask Suicide-Screening Questions (ASQ) Toolkit

Suicide Risk Screening Training: How to Manage Patients at Risk for Suicide
This video is provided for general informational purposes only and does not constitute an endorsement by NIMH. Webinar for Nurses - How to Use the ASQ to Detect Patients at Risk for Suicide
This video is provided for general informational purposes only and does not constitute an endorsement by NIMH. Universal Screening in the Emergency Department
This video is provided for general informational purposes only and does not constitute an endorsement by NIMH. Suicide Risk Screening Training for Nurses: How to Use the ASQ to Detect Patients at Risk for Suicide
This video is provided for general informational purposes only and does not constitute an endorsement by NIMH.
The Ask Suicide-Screening Questions (ASQ) tool is a brief validated tool for use among both youth and adults. The Joint Commission approves the use of the ASQ for all ages. Additional materials to help with suicide risk screening implementation are available in The Ask Suicide-Screening Questions (ASQ) Toolkit, a free resource for use in medical settings (emergency department, inpatient medical/surgical units, outpatient clinics/primary care) that can help providers successfully identify individuals at risk for suicide . The ASQ toolkit consists of youth and adult versions as some of the materials take into account developmental considerations.
The ASQ is a set of four screening questions that takes 20 seconds to administer. In an NIMH study , a “yes” response to one or more of the four questions identified 97% of youth (aged 10 to 21 years) at risk for suicide. Led by the NIMH, a multisite research study has now demonstrated that the ASQ is also a valid screening tool for adult medical patients. By enabling early identification and assessment of medical patients at high risk for suicide, the ASQ toolkit can play a key role in suicide prevention.
Suicide is a global public health problem and a leading cause of death across age groups worldwide. Suicide is also a major public health concern in the United States, with suicide ranking as the second leading cause of death among young people ages 10-24. According to the Centers for Disease Control and Prevention (CDC), more than 47,000 individuals killed themselves in 2019 . Even more common than death by suicide are suicide attempts and suicidal thoughts.
Screening for Suicide Risk
Early detection is a critical prevention strategy. The majority of people who die by suicide visit a healthcare provider within months before their death. This represents a tremendous opportunity to identify those at risk and connect them with mental health resources. Yet, most healthcare settings do not screen for suicide risk. In February 2016, the Joint Commission, the accrediting organization for health care programs in hospitals throughout the United States, issued a Sentinel Event Alert recommending that all medical patients in all medical settings (inpatient hospital units, outpatient practices, emergency departments) be screened for suicide risk. Using valid suicide risk screening tools that have been tested in the medical setting and with youth, will help clinicians accurately detect who is at risk and who needs further intervention.
Using an evidence-based clinical pathway can guide the process of identifying patients at risk and managing those who screen positive. Having a pathway to follow will save time and resources when responding to a positive screen. The ASQ Toolkit has several suicide risk clinical pathways that are built on the following foundation:
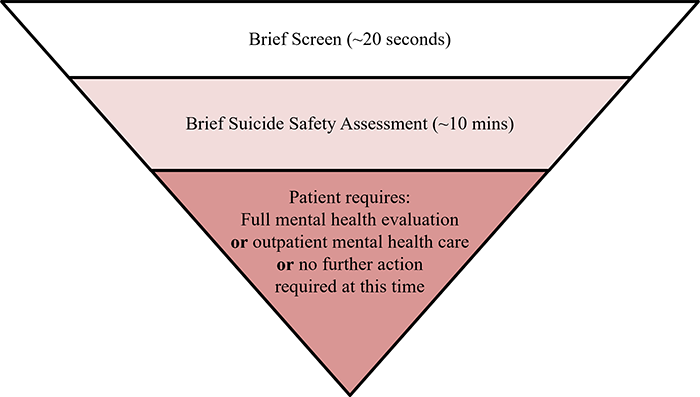
About the Tool
Beginning in 2008, NIMH led a multisite study to develop and validate a suicide risk screening tool for youth in the medical setting called the Ask Suicide-Screening Questions (ASQ). In 2014 another multisite research study was launched to validate the ASQ among adults. The ASQ consists of four yes/no questions and takes only 20 seconds to administer. Screening identifies individuals that require further mental health/suicide safety assessment.
For medical settings, one of the biggest barriers to screening is how to effectively and efficiently manage the patients that screen positive. Prior to screening for suicide risk, each setting will need to have a plan in place to manage patients that screen positive. The ASQ Toolkit was developed to assist with this management plan and to aid implementation of suicide risk screening and provide tools for the management of patients who are found to be at risk.
Using the Toolkit
The Ask Suicide-Screening Questions (ASQ) toolkit is designed to screen medical patients ages 8 years and above for risk of suicide. As there are no tools validated for use in kids under the age of 8 years, if suicide risk is suspected in younger children a full mental health evaluation is recommended instead of screening. The ASQ is free of charge and available in multiple languages.
For screening youth, it is recommended that screening be conducted without the parent/guardian present. Refer to the nursing script for guidance on requesting that the parent/guardian leave the room during screening. If the parent/guardian refuses to leave or the child insists that they stay, conduct the screening with the parent/guardian present. For all patients, any other visitors in the room should be asked to leave the room during screening.
What happens if patients screen positive?
Patients who screen positive for suicide risk on the ASQ should receive a brief suicide safety assessment (BSSA) conducted by a trained clinician (e.g., social worker, nurse practitioner, physician assistant, physician, or other mental health clinicians) to determine if a more comprehensive mental health evaluation is needed. The BSSA should be brief and guides what happens next in each setting. Any patient that screens positive, regardless of disposition, should be given the Patient Resource List .
The ASQ toolkit is organized by the medical setting in which it will be used: emergency department, inpatient medical/surgical unit, and outpatient primary care and specialty clinics. For questions regarding toolkit materials or implementing suicide risk screening, please contact: Lisa Horowitz, PhD, MPH at [email protected] or Debbie Snyder, MSW at [email protected] .
Youth Emergency Department (ED/ER) Inpatient Medical/Surgical Unit Outpatient Primary Care/Specialty Clinics
Adults Emergency Department (ED/ER) Inpatient Medical/Surgical Unit Outpatient Primary Care/Specialty Clinics
*Note: The following materials remain the same across all medical settings. These materials can be used in other settings with youth (e.g. school nursing office, juvenile detention centers).
- ASQ Information Sheet ( PDF | HTML )
- ASQ Tool ( PDF | HTML )
- ASQ in other languages
- Patient Resource List ( PDF | HTML )
- Educational Videos ( PDF | HTML )
- PHQ-A+ASQ ( PDF | HTML )
- PHQ-9+ASQ (PDF | HTML)
- Amharic ( PDF )
- Arabic ( PDF )
- Catalan ( PDF )
- Chinese ( PDF )
- Dutch ( PDF )
- Estonian ( PDF )
- Filipino ( PDF )
- French ( PDF )
- Hebrew ( PDF )
- Hindi ( PDF )
- Hungarian ( PDF )
- Italian ( PDF )
- Japanese ( PDF )
- Korean ( PDF )
- Nepali ( PDF )
- Portuguese ( PDF )
- European Portuguese ( PDF )
- Russian ( PDF )
- Somali ( PDF )
- Spanish ( PDF )
- Turkish ( PDF )
- Urdu ( PDF )
- Vietnamese ( PDF )
Suicide Prevention Resources
National Suicide Prevention Lifeline 1-800-273-TALK (8255) Spanish/español: 1-888-628-9454
Crisis Text Line Text HOME to 741-741
Suicide Prevention Resource Center
National Institute of Mental Health
Substance Abuse and Mental Health Services Administration
Horowitz, L. M., Bridge, J. A., Teach, S. J., Ballard, E., Klima, J., Rosenstein, D. L., ... & Pao, M. (2012). Ask Suicide-Screening Questions (ASQ): a brief instrument for the pediatric emergency department . Archives of Pediatrics & Adolescent Medicine, 166 (12), 1170-1176.
Horowitz, L. M., Snyder, D. J., Boudreaux, E. D., He, J. P., Harrington, C. J., Cai, J., Claassen, C. A., Salhany, J. E., Dao, T., Chaves, J. F., Jobes, D. A., Merikangas, K. R., Bridge, J. A., Pao, M. (2020). Validation of the Ask Suicide-Screening Questions (ASQ) for adult medical inpatients: A brief tool for all ages. Psychosomatics, 61 (6), 713-722.
Horowitz, L. M., Wharff, E. A., Mournet, A. M., Ross, A. M., McBee-Strayer, S., He, J., Lanzillo, E., White, E., Bergdoll, E., Powell, D. S., Merikangas, K. R., Pao, M., & Bridge, J. A. (2020). Validation and feasibility of the Ask Suicide-Screening Questions (ASQ) among pediatric medical/surgical inpatients. Hospital Pediatrics, 10 (9), 750-757
Aguinaldo, L. D., Sullivant, S., Lanzillo, E. C., Ross, A., He, J. P., Bradley-Ewing, A., Bridge, J. A., Horowitz, L. M., & Wharff, E. A. (2021). Validation of the Ask Suicide-Screening Questions (ASQ) with youth in outpatient specialty and primary care clinics . General Hospital Psychiatry, 68 , 52–58.
Brahmbhatt, K., Kurtz, B. P., Afzal, K. I., Giles, L. L., Kowal, E. D., Johnson, K. P., ... & Workgroup, P. (2019). Suicide risk screening in pediatric hospitals: Clinical pathways to address a global health crisis . Psychosomatics, 60 (1), 1-9.
Roaten, K., Horowitz, L. M., Bridge, J. A., Goans, C. R. R., McKintosh, C., Genzel, R., Johnson, C., & North, C. S. (2021). Universal pediatric suicide risk screening in a health care system: 90,000 patient encounters. Journal of the Academy of Consultation-Liaison Psychiatry.
Horowitz, L. M., Mournet, A. M., Lanzillo, E., He, J. P., Powell, D. S., Ross, A. M., Wharff, E. A., Bridge, J. A., & Pao, M. (2021). Screening pediatric medical patients for suicide risk: Is depression screening enough? Journal of Adolescent Health, S1054-139X(21)00060-4.
Mournet, A. M., Smith, J. T., Bridge, J. A., Boudreaux, E. D., Snyder, D. J., Claassen, C. A., Jobes, D. A, Pao, M., & Horowitz, L. M. (2021). Limitations of screening for depression as a proxy for suicide risk in adult medical inpatients. Journal of the Academy of Consultation-Liaison Psychiatry.
Thom, R., Hogan, C., & Hazen, E. (2020). Suicide Risk Screening in the Hospital Setting: A Review of Brief Validated Tools. Psychosomatics, 61 (1), 1–7.
Lanzillo, E. C., Horowitz, L. M., Wharff, E. A., Sheftall, A. H., Pao, M., & Bridge, J. A. (2019). The importance of screening preteens for suicide risk in the emergency department. Hospital Pediatrics, 9 (4), 305–307.
DeVylder, J. E., Ryan, T. C., Cwik, M., Wilson, M. E., Jay, S., Nestadt, P. S., Goldstein, M., & Wilcox, H. C. (2019). Assessment of selective and universal screening for suicide risk in a pediatric emergency department. JAMA Network Open, 2 (10), e1914070.
Ballard, E. D., Cwik, M., Van Eck, K., Goldstein, M., Alfes, C., Wilson, M. E., ... & Wilcox, H. C. (2017). Identification of at-risk youth by suicide screening in a pediatric emergency department . Prevention Science, 18 (2), 174-182.
Newton, A. S., Soleimani, A., Kirkland, S. W., & Gokiert, R. J. (2017). A systematic review of instruments to identify mental health and substance use problems among children in the emergency department . Academic Emergency Medicine, 24 (5), 552-568.
Ross, A. M., White, E., Powell, D., Nelson, S., Horowitz, L., & Wharff, E. (2016). To ask or not to ask? Opinions of pediatric medical inpatients about suicide risk screening in the hospital . The Journal of Pediatrics, 170 , 295-300.
Horowitz, L. M., Bridge, J. A., Pao, M., & Boudreaux, E. D. (2014). Screening youth for suicide risk in medical settings: time to ask questions . American Journal of Preventive Medicine, 47 (3), S170-S175.
Ballard, E. D., Bosk, A., Pao, M., Snyder, D., Bridge, J. A., Wharff, E. A., Teach, S. J., & Horowitz, L. (2012). Patients’ opinions about suicide screening in a pediatric emergency department . Pediatric Emergency Care, 28 (1), 34.

IMAGES
VIDEO
COMMENTS
Ethical guidelines are established for clinical research to protect patient volunteers and to preserve the integrity of the science. NIH Clinical Center researchers published seven main principles to guide the conduct of ethical research: Social and clinical value. Scientific validity.
This comprehensive guide to health research reaches out to a wide spectrum of people: students who wish to learn the basic principles of health research and how to conduct it, field researchers, and those involved in teaching and training of health research methodologies. It seeks to develop practical skills, starting with defining the ...
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. ... International ethical guidelines for biomedical ...
The NIH and other Federal agencies have developed policies, regulations, and guidelines for investigators to follow for conducting safe, ethical, and high-quality clinical research. This page provides information that includes but is not limited to federal and NIH human subjects research policies and guidelines for monitoring clinical research ...
Introduction. Good Clinical Research Practice (GCP) is a process that incorporates established ethical and scientifi c quality standards for the design, conduct, recording and reporting of clinical research involving the participation of human subjects. Compliance with GCP provides public assurance that the rights, safety, and well-being of ...
JAMA. Review. December 12, 2022. This Guide to Statistics and Methods describes the use of target trial emulation to design an observational study so it preserves the advantages of a randomized clinical trial, points out the limitations of the method, and provides an example of its use. Research, Methods, Statistics.
October 19, 2021. This Users' Guide to the Medical Literature provides suggestions for understanding guideline methods and recommendations for clinicians seeking direction in evaluating clinical practice guidelines for potential use in their practice. Guidelines Conflict of Interest.
Guidance Title Topic Draft or Final Date Issued; Informed Consent: Good Clinical Practice (GCP) Final: 8/15/2023: Decentralized Clinical Trials for Drugs, Biological Products, and Devices
Medical Research Council July 2012. RC ethics seriesGood research practice: Principles and guidelinesThe Medical Research Council (MRC) is ded. cated to improving human health through excellent medical research. The MRC expects that the research it supports is conducted according to the highest achievable standards of research pr.
ISBN: 9780781768993. Publication Date: 2009-06-08. "CTP" has been established as the cornerstone text in the field of psychiatry and mental health. This Ninth Edition provides updated information in neural science, genetics, neuropsychiatry, psychopharmacotherapy, and other key areas.
Abstract. This comprehensive guide to health research reaches out to a wide spectrum of people: students who wish to learn the basic principles of health research and how to conduct it, field researchers, and those involved in teaching and training of health research methodologies. It seeks to develop practical skills, starting with defining ...
Principles of Research Methodology: A Guide for Clinical Investigators is the definitive, comprehensive guide to understanding and performing clinical research. Designed for medical students, physicians, basic scientists involved in translational research, and other health professionals, this indispensable reference also addresses the unique challenges and demands of clinical research and ...
Research, first issued in February 1988. Clinical research may be defined as investigations involving human subjects or the use of patient samples. The scientific practices described here are generally accepted by investigators conducting both multi-center and single-institution clinical studies and help ensure both the quality and integrity of ...
The term evidence-based medicine (EBM) refers to the practice of caring for patients using the best available research evidence to guide clinical decision-making ( figure 1) [ 1,2 ]. The value of EBM is heightened in light of the following considerations:
clinical research handbook will be available for physicians and PIs starting in January 2021. This. clinical handbook starts by discussing various ways for the clinical studies to be organized and. executed, including a step-by-step approach to research documentation while managing. regulatory and ethical concerns in research.
See all COVID-19 research updates, including updated human-subjects research guidance and participant screening script, here. This clinical research guidebook has been developed for faculty and staff members engaged in clinical research at Penn State College of Medicine/Penn State Health Milton S. Hershey Medical Center.
Campbell University's medical librarian is happy to assist you with selecting and narrowing a topic, formulating a clinical question, selecting databases, constructing and executing a literature search, and locating articles. You can contact the medical library at either 910-893-7700 during business hours, or [email protected].
The purpose of having reporting guidelines in medical research is to create a manual for the authors to follow, which should lead to total transparency, accurate reporting, and easier assessment of the validity of reported research findings. This goal has been reached to some degree, but it is still necessary to be critical when appraising any ...
Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials. ... Recommendations and guidelines are available for clinical studies (14, 20, e10, e11), for diagnostic studies ...
My first full length book is called "The Comprehensive Guide To Clinical Research". Throughout my career I have worked at the site, CRO, Sponsor and vendor levels of clinical research in some capacity. As a former study coordinator and current contract CRA and Site Owner, I am uniquely positioned to have a holistic perspective when it comes to ...
Medical research involves research in a wide range of fields, such as biology, chemistry, pharmacology and toxicology with the goal of developing new medicines or medical procedures or improving ...
"Clinical practice guidelines are systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances."(Institute of Medicine, 1990) Issued by third-party organizations, and not NCCIH, these guidelines define the role of specific diagnostic and treatment modalities in the diagnosis and management of patients.
Introduction. This two-part series will present basic statistical principles for the practicing physician to use in his or her review of the literature and to the physician engaged in clinical research. The purpose of this series is threefold: (1) to provide an overview of common epidemiological and statistical terms and concepts that can be ...
This Guideline on Clinical Trials and Biomedical Research sets the ethical principles and the code of professional conduct for clinical trials involving human subjects, and outlines the role and functions of an institutional Ethical Committee in regulating the research. BRIEF HISTORY. 1.1 Biomedical experimentation using human subjects had ...
Double your impact on fighting cancer. Make a gift before July 31 and it can go twice as far to fight cancer. Explore comprehensive guides on hundreds of common and rare diseases and conditions from the experts at Mayo Clinic.
Journal metrics Editorial board. Current Medical Research and Opinion (CMRO) is a MEDLINE-indexed, international journal that publishes research focused on new and existing drugs and therapies, best practices in patient care, developments in diagnostic medicine and medical technology, and innovations in medical and scientific publishing.
The goal of the Grants for Early Medical/Surgical Specialists' Transition to Aging Research (GEMSSTAR) program is to provide support for early-career physician-scientists trained in medical or surgical specialties and early-career dentist-scientists to launch careers as future leaders in aging- or geriatric-focused research.
The Clinician's Guide offers concise recommendations regarding prevention, risk assessment, diagnosis, and treatment of osteoporosis in postmenopausal women and men aged 50 years and older. It includes indications for bone densitometry as well as fracture risk thresholds for pharmacologic intervention. Current medications build bone and/or ...
Author Guidelines. Medical Education is an international peer-reviewed journal with distribution to readers in more than 80 countries. The journal seeks to enhance its position as the pre-eminent journal in the field of education for healthcare professionals and aims to publish material of the highest quality reflecting worldwide or provocative issues and perspectives.
Using valid suicide risk screening tools that have been tested in the medical setting and with youth, will help clinicians accurately detect who is at risk and who needs further intervention. Using an evidence-based clinical pathway can guide the process of identifying patients at risk and managing those who screen positive.