
- Previous Article
- Next Article

Case Presentation
Case study: a patient with diabetes and weight-loss surgery.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Sue Cummings; Case Study: A Patient With Diabetes and Weight-Loss Surgery. Diabetes Spectr 1 July 2007; 20 (3): 173–176. https://doi.org/10.2337/diaspect.20.3.173
Download citation file:
- Ris (Zotero)
- Reference Manager
A.W. is a 65-year-old man with type 2 diabetes who was referred by his primary care physician to the weight center for an evaluation of his obesity and recommendations for treatment options, including weight-loss surgery. The weight center has a team of obesity specialists, including an internist, a registered dietitian (RD), and a psychologist, who perform a comprehensive initial evaluation and make recommendations for obesity treatment. A.W. presented to the weight center team reluctant to consider weight-loss surgery;he is a radiologist and has seen patients who have had complications from bariatric surgery.
Pertinent medical history. A.W.'s current medications include 30 and 70 units of NPH insulin before breakfast and before or after dinner, respectively, 850 mg of metformin twice daily, atorvastatin,lisinopril, nifedipine, allopurinol, aspirin, and an over-the-counter vitamin B 12 supplement. He has sleep apnea but is not using his continuous positive airway pressure machine. He reports that his morning blood glucose levels are 100–130 mg/dl, his hemoglobin A 1c (A1C) level is 6.1%, which is within normal limits, his triglyceride level is 201 mg/dl, and serum insulin is 19 ulU/ml. He weighs 343 lb and is 72 inches tall, giving him a BMI of 46.6 kg/m 2 .
Weight history. A.W. developed obesity as a child and reports having gained weight every decade. He is at his highest adult weight with no indication that medications or medical complications contributed to his obesity. His family history is positive for obesity; his father and one sister are also obese.
Dieting history. A.W. has participated in both commercial and medical weight-loss programs but has regained any weight lost within months of discontinuing the programs. He has seen an RD for weight loss in the past and has also participated in a hospital-based, dietitian-led, group weight-loss program in which he lost some weight but regained it all. He has tried many self-directed diets, but has had no significant weight losses with these.
Food intake. A.W. eats three meals a day. Dinner, his largest meal of the day, is at 7:30 p . m . He usually does not plan a mid-afternoon snack but will eat food if it is left over from work meetings. He also eats an evening snack to avoid hypoglycemia. He reports eating in restaurants two or three times a week but says his fast-food consumption is limited to an occasional breakfast sandwich from Dunkin'Donuts. His alcohol intake consists of only an occasional glass of wine. He reports binge eating (described as eating an entire large package of cookies or a large amount of food at work lunches even if he is not hungry) about once a month, and says it is triggered by stress.
Social history. Recently divorced, A.W. is feeling depressed about his life situation and has financial problems and stressful changes occurring at work. He recently started living with his girlfriend, who does all of the cooking and grocery shopping for their household.
Motivation for weight loss. A.W. says he is concerned about his health and wants to get his life back under control. His girlfriend, who is thin and a healthy eater, has also been concerned about his weight. His primary care physician has been encouraging him to explore weight-loss surgery; he is now willing to learn more about surgical options. He says that if the weight center team's primary recommendation is for weight-loss surgery,he will consider it.
Does A.W. have contraindications to weight-loss surgery, and, if not, does he meet the criteria for weight-loss surgery?
What type of weight-loss surgery would be best for A.W.?
Roles of the obesity specialist team members
The role of the physician as an obesity specialist is to identify and evaluate obesity-related comorbidities and to exclude medically treatable causes of obesity. The physician assesses any need to adjust medications and,if possible, determines if the patient is on a weight-promoting medication that may be switched to a less weight-promoting medication.
The psychologist evaluates weight-loss surgery candidates for a multitude of factors, including the impact of weight on functioning, current psychological symptoms and stressors, psychosocial history, eating disorders,patients' treatment preferences and expectations, motivation, interpersonal consequences of weight loss, and issues of adherence to medical therapies.
The RD conducts a nutritional evaluation, which incorporates anthropometric measurements including height (every 5 years), weight (using standardized techniques and involving scales in a private location that can measure patients who weigh > 350 lb), neck circumference (a screening tool for sleep apnea), and waist circumference for patients with a BMI < 35 kg/m 2 . Other assessments include family weight history,environmental influences, eating patterns, and the nutritional quality of the diet. A thorough weight and dieting history is taken, including age of onset of overweight or obesity, highest and lowest adult weight, usual weight, types of diets and/or previous weight-loss medications, and the amount of weight lost and regained with each attempt. 1
Importance of type of obesity
Childhood- and adolescent-onset obesity lead to hyperplasic obesity (large numbers of fat cells); patients presenting with hyperplasic and hypertrophic obesity (large-sized fat cells), as opposed to patients with hypertrophic obesity alone, are less likely to be able to maintain a BMI < 25 kg/m 2 , because fat cells can only be shrunk and not eliminated. This is true even after weight-loss surgery and may contribute to the variability in weight loss outcomes after weight loss surgery. Less than 5% of patients lose 100% of their excess body weight. 2 , 3
Criteria and contraindications for weight-loss surgery
In 1998, the “Clinical Guidelines on the Identification, Evaluation,and Treatment of Overweight and Obesity in Adults: The Evidence Report” 4 recommended that weight-loss surgery be considered an option for carefully selected patients:
with clinically severe obesity (BMI ≥ 40 kg/m 2 or ≥ 35 kg/m 2 with comorbid conditions);
when less invasive methods of weight loss have failed; and
the patient is at high risk for obesity-associated morbidity or mortality.
Contraindications for weight-loss surgery include end-stage lung disease,unstable cardiovascular disease, multi-organ failure, gastric verices,uncontrolled psychiatric disorders, ongoing substance abuse, and noncompliance with current regimens.
A.W. had no contraindications for surgery and met the criteria for surgery,with a BMI of 46.6 kg/m 2 . He had made numerous previous attempts at weight loss, and he had obesity-related comorbidities, including diabetes,sleep apnea, hypertension, and hypercholesterolemia.
Types of procedures
The roux-en-Y gastric bypass (RYGB) surgery is the most common weight-loss procedure performed in the United States. However, the laparoscopic adjustable gastric band (LAGB) procedure has been gaining popularity among surgeons. Both procedures are restrictive, with no malabsorption of macronutrients. There is,however, malabsorption of micronutrients with the RYGB resulting from the bypassing of a major portion of the stomach and duodenum. The bypassed portion of the stomach produces the intrinsic factor needed for the absorption of vitamin B 12 . The duodenum is where many of the fat-soluble vitamins, B vitamins, calcium, and iron are absorbed. Patients undergoing RYGB must agree to take daily vitamin and mineral supplementation and to have yearly monitoring of nutritional status for life.
Weight loss after RYGB and LAGB
The goal of weight-loss surgery is to achieve and maintain a healthier body weight. Mean weight loss 2 years after gastric bypass is ∼ 65% of excess weight loss (EWL), which is defined as the number of pounds lost divided by the pounds of overweight before surgery. 5 When reviewing studies of weight-loss procedures, it is important to know whether EWL or total body weight loss is being measured. EWL is about double the percentage of total body weight loss; a 65% EWL represents about 32% loss of total body weight.
Most of the weight loss occurs in the first 6 months after surgery, with a continuation of gradual loss throughout the first 18–24 months. Many patients will regain 10–15% of the lost weight; a small number of patients regain a significant portion of their lost weight. 6 Data on long-term weight maintenance after surgery indicate that if weight loss has been maintained for 5 years, there is a > 95% likelihood that the patient will keep the weight off over the long term.
The mean percentage of EWL for LAGB is 47.5%. 3 Although the LAGB is considered a lower-risk surgery, initial weight loss and health benefits from the procedure are also lower than those of RYGB.
Weight-loss surgery and diabetes
After gastric bypass surgery, there is evidence of resolution of type 2 diabetes in some individuals, which has led some to suggest that surgery is a cure. 7 Two published studies by Schauer et al. 8 and Sugarman et al. 9 reported resolution in 83 and 86% of patients, respectively. Sjoström et al. 10 published 2-and 10-year data from the Swedish Obese Subjects (SOS) study of 4,047 morbidly obese subjects who underwent bariatric surgery and matched control subjects. At the end of 2 years, the incidence of diabetes in subjects who underwent bariatric surgery was 1.0%, compared to 8.0% in the control subjects. At 10 years, the incidence was 7.0 and 24.0%, respectively.
The resolution of diabetes often occurs before marked weight loss is achieved, often days after the surgery. Resolution of diabetes is more prevalent after gastric bypass than after gastric banding (83.7% for gastric bypass and 47.9% for gastric banding). 5 The LAGB requires adjusting (filling the band through a port placed under the skin),usually five to six times per year. Meta-analysis of available data shows slower weight loss and less improvement in comorbidities including diabetes compared to RYGB. 5
A.W. had diabetes; therefore, the weight center team recommended the RYGB procedure.
Case study follow-up
A.W. had strong medical indications for surgery and met all other criteria outlined in current guidelines. 4 He attended a surgical orientation session that described his surgical options,reviewed the procedures (including their risks and possible complications),and provided him the opportunity to ask questions. This orientation was led by an RD, with surgeons and post–weight-loss surgical patients available to answer questions. After attending the orientation, A.W. felt better informed about the surgery and motivated to pursue this treatment.
The weight center evaluation team referred him to the surgeon for surgical evaluation. The surgeon agreed with the recommendation for RYGB surgery, and presurgical appointments and the surgery date were set. The surgeon encouraged A.W. to try to lose weight before surgery. 11
Immediately post-surgery. The surgery went well. A.W.'s blood glucose levels on postoperative day 2 were 156 mg/dl at 9:15 a . m . and 147 mg/dl at 11:15 a . m . He was discharged from the hospital on that day on no diabetes medications and encouraged to follow a Stage II clear and full liquid diet( Table 1 ). 12
Diet Stages After RYBG Surgery

On postoperative day 10, he returned to the weight center. He reported consuming 16 oz of Lactaid milk mixed with sugar-free Carnation Instant Breakfast and 8 oz of light yogurt, spread out over three to six meals per day. In addition, he was consuming 24 oz per day of clear liquids containing no sugar, calories, or carbonation. A.W.'s diet was advanced to Stage III,which included soft foods consisting primarily of protein sources (diced,ground, moist meat, fish, or poultry; beans; and/or dairy) and well-cooked vegetables. He also attended a nutrition group every 3 weeks, at which the RD assisted him in advancing his diet.
Two months post-surgery. A.W. was recovering well; he denied nausea, vomiting, diarrhea, or constipation. He was eating without difficulty and reported feeling no hunger. His fasting and pre-dinner blood glucose levels were consistently < 120 mg/dl, with no diabetes medications. He continued on allopurinol and atorvastatin and was taking a chewable daily multivitamin and chewable calcium citrate (1,000 mg/day in divided doses) with vitamin D (400 units). His weight was 293 lb, down 50 lb since the surgery. A pathology report from a liver biopsy showed mild to moderate steatatosis without hepatitis.
One year post-surgery. A.W.'s weight was 265 lb, down 78 lb since the surgery, and his weight loss had significantly slowed, as expected. He was no longer taking nifedipine or lisinipril but was restarted at 5 mg daily to achieve a systolic blood pressure < 120 mmHg. His atorvastatin was stopped because his blood lipid levels were appropriate (total cholesterol 117 mg/dl, triglycerides 77 mg/dl, HDL cholesterol 55 mg/dl, and LDL cholesterol 47 mg/dl). His gastroesophageal reflux disease has been resolved, and he continued on allopurinol for gout but had had no flare-ups since surgery. Knee pain caused by osteoarthritis was well controlled without anti-inflammatory medications, and he had no evidence of sleep apnea. Annual medical follow-up and nutritional laboratory measurements will include electrolytes, glucose,A1C, albumin, total protein, complete blood count, ferritin, iron, total iron binding capacity, calcium, parathyroid hormone, vitamin D, magnesium, vitamins B 1 and B 12 , and folate, as well as thyroid, liver, and kidney function tests and lipid measurements.
In summary, A.W. significantly benefited from undergoing RYBP surgery. By 1 year post-surgery, his BMI had decreased from 46.6 to 35.8 kg/m 2 ,and he continues to lose weight at a rate of ∼ 2 lb per month. His diabetes, sleep apnea, and hypercholesterolemia were resolved and he was able to control his blood pressure with one medication.
Clinical Pearls
Individuals considering weight loss surgery require rigorous presurgical evaluation, education, and preparation, as well as a comprehensive long-term postoperative program of surgical, medical, nutritional, and psychological follow-up.
Individuals with diabetes should consider the RYBP procedure because the data on resolution or significant improvement of diabetes after this procedure are very strong, and such improvements occur immediately. Resolution in or improvement of diabetes with the LAGB procedure are more likely to occur only after excess weight has been lost.
Individuals with diabetes undergoing weight loss surgery should be closely monitored; an inpatient protocol should be written regarding insulin regimens and sliding-scale use of insulin if needed. Patients should be educated regarding self-monitoring of blood glucose and the signs and symptoms of hypoglycemia. They should be given instructions on stopping or reducing medications as blood glucose levels normalize.
Patient undergoing RYGB must have lifetime multivitamin supplementation,including vitamins B 1 , B 12 , and D, biotin, and iron, as well as a calcium citrate supplement containing vitamin D (1,000–1,500 mg calcium per day). Nutritional laboratory measurements should be conducted yearly and deficiencies repleted as indicated for the duration of the patient's life.
Sue Cummings, MS, RD, LDN, is the clinical programs coordinator at the MGH Weight Center in Boston, Mass.
Email alerts
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Dermatology
- Gastroenterology
- Geriatric Medicine and Gerontology
- Gynecology and Obstetrics
- Heart and Vascular
- Neurology and Neurosurgery
- Ophthalmology
- Orthopaedics
- Otolaryngology–Head and Neck Surgery
- Physical Medicine and Rehabilitation
- Plastic and Reconstructive Surgery
- Psychiatry and Behavioral Sciences
- Pediatric Specialties
- Pediatric Diabetes and Endocrinology
- Pediatrics Florida
- Pediatric Gastroenterology and GI Surgery
- Pediatric Heart
- Pediatrics Maryland/DC
- Pediatric Neurology & Neurosurgery
- Pediatric Orthopaedics
- Physician Affiliations
- Health Care Technology
- High-Value Health Care
- Clinical Research Advancements
- Precision Medicine Excellence
- Patient Safety
Bariatric Surgery Case Study – Gastric Bypass with ICG Leak Test
Johns Hopkins Center for Bariatric Surgery, National Capital Region
Patient presentation
- A 38-year-old female with a history of class 3 obesity (BMI 45.9), gastroesophageal reflux disease (GERD), hypertension and sleep apnea presented with multiple failed attempts at medical weight-loss. She was initially interested in a minimally invasive sleeve gastrectomy, but a gastric bypass was recommended due to her history of GERD. A sleeve gastrectomy can worsen heartburn postoperatively, but a gastric bypass is a surgical treatment for both morbid obesity as well as GERD. The patient was evaluated by the bariatric multidisciplinary team at Sibley Memorial Hospital and approved for surgery.
Treatments received
- The patient underwent a minimally invasive Roux-en-Y gastric bypass using the latest camera technology. After her procedure, a new technique was used to test the gastro-jejunal anastomosis for any signs of a leak. A novel fluid solution containing indocyanine green dye was instilled into the stomach, and a laparoscopic camera with near-infrared fluorescence visualization was used to transilluminate the anastomosis. It gave real-time feedback and confirmed no leak was present.
Patient outcome after surgery
- The patient was kept overnight and discharged the following day after passing an oral fluid challenge. She was seen two weeks later and was feeling well, tolerating a soft diet and already beginning to see weight loss results.
Johns Hopkins Center for Bariatric Surgery at Sibley Memorial Hospital in Washington, D.C., is accredited from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) as a Comprehensive Center with Adult Qualifications. The team performs open and minimally invasive surgery using state-of-the art equipment. In addition to surgery, the multidisciplinary team provides nutrition counseling, exercise training and close follow-up after surgery.

- About Johns Hopkins Medicine
- Contact Johns Hopkins Medicine
- Centers & Departments
- Maps & Directions
- Find a Doctor
- Patient Care
- Terms & Conditions of Use
- Privacy Statement
Connect with Johns Hopkins Medicine
Join Our Social Media Communities >
Clinical Connection
- Otolaryngology—Head and Neck Surgery
- Contact Johns Hopkins
© The Johns Hopkins University, The Johns Hopkins Hospital, and Johns Hopkins Health System. All rights reserved.
Privacy Policy and Disclaimer
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Long term outcomes of...
Long term outcomes of metabolic/bariatric surgery in adults
- Related content
- Peer review
- Anita P Courcoulas , professor of surgery 1 ,
- Christopher R Daigle , service line medical director 2 ,
- David E Arterburn , senior investigator , affiliate professor 3 4
- 1 Department of Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
- 2 Bariatric Surgery Program, Washington Permanente Medical Group, Bellevue, WA, USA
- 3 Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA
- 4 Department of Medicine, University of Washington, Seattle, WA, USA
- Correspondence to: D E Arterburn David.E.Arterburn{at}kp.org
The prevalence of obesity continues to rise around the world, driving up the need for effective and durable treatments. The field of metabolic/bariatric surgery has grown rapidly in the past 25 years, with observational studies and randomized controlled trials investigating a broad range of long term outcomes. Metabolic/bariatric surgery results in durable and significant weight loss and improvements in comorbid conditions, including type 2 diabetes. Observational studies show that metabolic/bariatric surgery is associated with a lower incidence of cardiovascular events, cancer, and death. Weight regain is a risk in a fraction of patients, and an association exists between metabolic/bariatric surgery and an increased risk of developing substance and alcohol use disorders, suicidal ideation/attempts, and accidental death. Patients need lifelong follow-up to help to reduce the risk of these complications and other nutritional deficiencies. Different surgical procedures have important differences in risks and benefits, and a clear need exists for more long term research about less invasive and emerging procedures. Recent guidelines for the treatment of obesity and metabolic conditions have been updated to reflect this growth in knowledge, with an expansion of eligibility criteria, particularly people with type 2 diabetes and a body mass index between 30.0 and 34.9.
Introduction
Obesity is a well established risk factor for developing many chronic diseases, such as type 2 diabetes, cardiovascular disease, and cancer, as well as an increased risk of covid-19 related hospital admission and death. Despite these known risks, many patients, doctors, and health policy makers remain uncertain about the long term efficacy and safety of available treatments for obesity. Recent advances in drug therapy have increased the demand for obesity treatments, but long term (five years or more of follow-up) data on drug therapy for obesity have remained relatively scant. On the other hand, long term evidence on the efficacy and safety of metabolic/bariatric surgery (MBS) has continued to accrue over the past 25 years, particularly for adults with type 2 diabetes. Recent data highlight the durability of weight loss and improvements in comorbidity, and the comparative effectiveness of alternative approaches to MBS, but they also clearly indicate risks, including the potential for weight regain, reoperation, and a higher incidence of substance use disorders and suicide. Given the important trade-offs between the long term risks and benefits of MBS, doctors should engage patients with severe obesity in a shared decision making conversation about the role of MBS in the management of their weight and related health problems. This review summarizes recent and emerging evidence related to the safety, efficacy, and metabolic outcomes of MBS to help guide clinical decision making. We also alert clinicians to emerging trends in treatment and major unanswered research questions to help to guide conversations with patients.
Sources and selection criteria
We based this review on articles found by searching PubMed and the Cochrane Library from the time of our last review (2014) 1 to December 2022, with the terms “bariatric surgery”, “gastric bypass”, “sleeve gastrectomy”, “one anastomosis gastric bypass”, and “biliopancreatic diversion”. Our search was limited to English language articles. We gave priority to evidence obtained from systematic literature reviews, meta-analyses, and randomized controlled trials (RCTs) when possible. We sought to include studies that were at least one year and preferably five years or more in duration where possible. We excluded studies of MBS that did not include a control or comparator group, where possible.
The prevalence of obesity (commonly defined as body mass index (BMI) ≥30) has continued to increase, with 42.4% of adults in the US having obesity in 2017-18 and 9.2% of adults having severe obesity (BMI ≥40). 2 In England in 2017, 27.4% of adults had BMI ≥30 and 2.5% of adults had BMI ≥40. 3 In a report on the prevalence of obesity in 27 EU member states and Kuwait during 2018-20, 16.3% of adults had BMI ≥30 and rates of BMI ≥40 varied widely from 1.7% (Spain) to 5.5% (Kuwait). 4 5
In the US, 198 000 bariatric operations were performed in 2020, with 61.6% being sleeve gastrectomy, 20.7% Roux-en-y gastric bypass (RYGB), 1.2% adjustable gastric banding (AGB), 0.7% one anastomosis gastric bypass (OAGB), and 11.1% revisional operations. 6 Outside North America, more than 375 000 bariatric operations were performed from 2014 to 2018, including approximately 38% RYGB, 46% sleeve gastrectomy, 8% OAGB, and 5% AGB. 7 The popularity of AGB has declined rapidly worldwide in recent years, whereas sleeve gastrectomy has been rising rapidly and OAGB is increasingly performed.
All MBS procedures are now routinely carried out with a less invasive, laparoscopic approach. Figure 1 shows the four most common bariatric procedures. Sleeve gastrectomy is a stomach-only operation that is performed by mobilizing the greater curvature of the stomach from its attachments and then dividing the stomach vertically around a calibrated bougie that is 36 to 40 French in size. The transected part of the stomach, which consists of approximately two thirds to three quarters of the stomach, is removed, and the new remaining stomach is a long and narrow curved tube. RYGB is a stomach and small intestine operation that consists of a small divided proximal gastric pouch and a modest small intestinal bypass of approximately 100-150 cm in length. The common channel of small intestine beyond the bypass allows for adequate absorption of nutrients. The residual stomach is not removed but is a conduit for digestive secretions only. Biliopancreatic diversion with or without duodenal switch is a gastric sleeve stomach resection with a longer intestinal bypass component at the end of the stomach. Finally, AGB is a silicone ring that is wrapped around the upper stomach, just below the esophagus, with an inflatable inner balloon to adjust the amount of gastric restriction via an infusion port placed outside the abdominal cavity. Other less common and emerging procedures are reviewed later in this article.

Common metabolic/bariatric surgery procedures. A: sleeve gastrectomy; B: Roux-en-Y gastric bypass; C: biliopancreatic diversion; D: adjustable gastric banding. Adapted from American Society for Metabolic and Bariatric Surgery. Bariatric Surgery Procedures ( https://asmbs.org/patients/bariatric-surgery-procedures )
- Download figure
- Open in new tab
- Download powerpoint
Randomized controlled trials comparing bariatric surgery with medical and lifestyle therapy
In the past 15 years, 13 RCTs have compared MBS with lifestyle and medical therapy for the treatment of type 2 diabetes ( table 1 ), showing that MBS results in significantly larger short to mid-term improvements in glycemic control, disease remission, cardiovascular risk factors, and chronic kidney disease. Findings from randomized trials of MBS resulted in international diabetes organizations publishing a joint statement supporting the consideration of MBS as a treatment option for patients with a BMI of 30.0-34.9 and type 2 diabetes. 21
Randomized controlled trials of metabolic/bariatric surgery versus medical and lifestyle treatment for type 2 diabetes treatment
- View inline
The trial with the longest follow-up to date was from Italy and randomized 60 patients to medical therapy, RYGB, or biliopancreatic diversion. 20 At 10 years, type 2 diabetes remission rates were 5.5% (95% confidence interval 1.0% to 25.7%) for medical therapy (one participant went into remission after crossover to surgery), 50.0% (29.9% to 70.1%) for biliopancreatic diversion, and 25.0% (11.2% to 46.9%) for RYGB. 20 People in the RYGB and biliopancreatic diversion groups had fewer diabetes related complications than those in the medical group (relative risk 0.07 (95% confidence interval 0.01 to 0.48) for both MBS comparisons). Serious adverse events occurred more commonly among participants in the surgical groups. In the US, the STAMPEDE study published five year results of a three arm trial comparing intensive medical therapy with either RYGB or sleeve gastrectomy plus intensive medical therapy. Among 150 patients in that study with type 2 diabetes and a BMI of 27-43, MBS was more effective than intensive medical therapy alone. The primary endpoint of glycated hemoglobin ≤6.0% with or without the use of diabetes medications was met by two (5%) of 38 patients who had intensive medical therapy, compared with 14 (29%) of 49 patients who underwent RYGB (adjusted P=0.03; P=0.08 in the intention-to-treat analysis) and 11 (23%) of 47 patients who underwent sleeve gastrectomy (adjusted P=0.07; P=0.17 in the intention-to-treat analysis). 17 Patients who underwent the surgical procedures had a greater mean absolute reduction in glycated hemoglobin from baseline than did patients who had medical therapy alone (2.1% v 0.3%; P=0.003). The two surgical groups also had superior weight loss, better lipid concentrations, reduced insulin use, and higher quality of life scores. 17
The STAMPEDE study and 12 other randomized trials either included or were specifically designed to study a population with BMI <35 (class 1 obesity), and these results also show significant improvements in type 2 diabetes in the lower BMI group ( table 1 ). Four of these randomized trials merged to form the Alliance of Randomized Trials of Medicine versus Metabolic Surgery in Type 2 Diabetes (ARMMS-T2D) consortium that is collecting pooled, longitudinal follow-up data for participants previously randomly assigned to surgical versus non-surgical treatment of type 2 diabetes. This group of 316 participants now represents the largest cohort of patients (approximately one third with BMI 30-34.9) ever randomly assigned to surgical versus non-surgical treatments for type 2 diabetes. Three year results from ARMMS have been published and seven to 10 year results are anticipated. 16 At three years, remission of diabetes was achieved in more participants after surgery than with medical/lifestyle intervention (60/160 (37.5%) v 2/76 (2.6%); P<0.001). Reductions in glycated hemoglobin and BMI were also greater after surgery. 16
These RCTs have their limitations, including small sample sizes and, for most studies, inadequate duration to detect differences in the incidence of cardiovascular and end organ complications of type 2 diabetes. In addition, the definition of remission of diabetes varied between studies, as did the proportion of people with BMI <35 ( table 1 ). Finally, the type of non-surgical treatments (lifestyle, drugs, exercise) and adherence to the program varied between studies.
Steatotic liver disease
Steatotic liver disease is a new overarching term, now used to describe the various etiologies of steatosis/fatty liver disease. Non-alcoholic fatty liver disease is now labeled as metabolic dysfunction associated steatotic liver disease (MASLD) and includes patients who have hepatic steatosis and have at least one of five cardiometabolic risk factors. A new category, termed MetALD, now describes people with MASLD who consume greater amounts of alcohol per week, and metabolic dysfunction associated steatohepatitis (MASH) is the replacement term for non-alcoholic steatohepatitis. 22 Observational studies suggest that MBS may improve MASH. In a large, single center study, among people with MASH and obesity, MBS compared with non-surgical management was associated with a significantly lower risk of incident major adverse liver outcomes and major adverse cardiovascular events (MACE). Patients with MASH (n=1158: 650 who underwent MBS and 508 in a non-surgical control group) were followed for seven years. The cumulative incidence of major adverse liver outcomes at 10 years was 2.3% (0% to 4.6%) in the MBS group and 9.6% (6.1% to 12.9%) in the non-surgical group (adjusted absolute risk difference 12.4%, 95% confidence interval 5.7% to 19.7%; adjusted hazard ratio 0.12, 95% confidence interval 0.02 to 0.63; P=0.01). The cumulative incidence of MACE at 10 years was 8.5% (5.5% to 11.4%) in the bariatric surgery group and 15.7% (11.3% to 19.8%) in the non-surgical group (adjusted absolute risk difference 13.9%, 5.9% to 21.9%; adjusted hazard ratio 0.30, 0.12 to 0.72; P=0.007). 23 The efficacy of MBS on MASH has recently been compared with lifestyle interventions and medical therapy in a randomized trial in Italy in which 288 people with obesity and MASH were assigned to lifestyle modification plus best medical care, RYGB, or sleeve gastrectomy. Histological resolution of MASH without worsening of fibrosis at one year follow-up was significantly higher in the RYGB (54; 56%) and sleeve gastrectomy group (55; 57%) compared with lifestyle modification (15; 16%) (P<0.001). MASH resolution was 3.60 (95% confidence interval 2.19 to 5.92; P<0.001) times greater in the RYGB group and 3.67 (2.23 to 6.02; P<0.001) times greater in the sleeve gastrectomy group compared with the lifestyle group. 24 This trial complements the observational data and indicates that MBS may be more effective than lifestyle intervention for the treatment of MASH.
Cardiovascular disease, microvascular disease, and mortality
Cardiovascular disease is a leading cause of death among adults, particularly those with severe obesity. Although behavioral and pharmacological weight loss interventions can improve cardiovascular risk factors (for example, blood pressure and glycemic control) among adults with obesity, no studies have shown that non-surgical weight loss can reduce the incidence of major cardiovascular disease events. 25 On the other hand, we identified 10 observational studies involving nine separate cohorts with a total of >120 000 patients who had MBS ( table 2 ) that consistently show a significant association between MBS and a lower risk of primary or secondary cardiovascular disease events compared with non-surgical interventions or usual medical care, including several studies among adults with type 2 diabetes. A major limitation in this area is a lack of RCTs (owing to the high cost of conducting trials powered for cardiovascular disease endpoints); however, the magnitude of the effect sizes is so large in these observational studies that unmeasured confounders are unlikely to be driving this association. 35 36 Also, these studies have not compared MBS against newer non-surgical interventions, such as sodium-glucose cotransporter-2 inhibitors or glucagon-like peptide-1 receptor agonists, which have been shown to reduce the risk of cardiovascular events. Finally, up until 2021, most of the data have come from patients undergoing RYGB; however, two recent large observational studies suggest that sleeve gastrectomy may also be associated with better cardiovascular disease outcomes than usual non-surgical treatment. 33 34
Observational studies of metabolic/bariatric surgery (MBS) and cardiovascular disease (CVD) outcomes
Microvascular disease —As with cardiovascular disease outcomes, more evidence has emerged in recent years indicating that MBS may reduce the risk of microvascular complications of type 2 diabetes, such as nephropathy, retinopathy, and neuropathy. This includes limited data from three randomized trials comparing MBS with intensive medical-lifestyle treatment of type 2 diabetes and obesity, 17 20 37 which suggest that MBS may result in less microalbuminuria and greater improvement in estimated glomerular filtration rate ( table 3 ). We identified additional support for improvements in microvascular outcomes after MBS from two recent systematic reviews. The first identified two RCTs and 12 observational studies involving more than 110 000 patients receiving MBS that together estimated an 83% lower relative risk (0.17, 0.13 to 0.22) of diabetic retinopathy at a median of two years’ follow-up compared with non-surgical treatment. 38 The second involved three RCTs and seven observational studies including 3459 patients receiving MBS, which together suggested a 74% lower risk (odds ratio 0.26, 95% confidence interval 0.15 to 0.42) of developing any microvascular disease (composite of nephropathy, retinopathy, and neuropathy). 39 In both these systematic reviews, most of the data come from the retrospective observational studies.
Systematic reviews and randomized trials of metabolic/bariatric surgery (MBS) and microvascular disease outcomes
Mortality —Although numerous RCTs of MBS have been conducted, none has yet been powered to investigate its effect on long term mortality compared with non-surgical treatment. However, at least 32 observational studies have examined the effects of MBS on mortality (supplementary table 25 ) involving more than 173 000 patients receiving MBS, with a median relative reduction in mortality of 46% (range 16-89%). 25
Long term comparative outcomes of gastric bypass versus sleeve gastrectomy
Sleeve gastrectomy is now the most common bariatric procedure performed worldwide, and since our last review we identified eight new randomized trials with five years or longer follow-up examining differences in outcomes between RYGB and sleeve gastrectomy. 17 41 42 43 44 45 Some studies were designed to directly compare outcomes between the procedures, whereas others prospectively compared metabolic procedures head to head with intensive medical management. 17 41 42 43 46 In the STAMPEDE trial, involving 49 patients having RYGB and 47 having sleeve gastrectomy, RYGB showed better mean weight loss compared with sleeve gastrectomy (−23.2 (standard deviation 9.6) kg v −18.6 (7.5) kg; P=0.01), but no statistically significant differences in diabetes outcomes were seen. 17 47 In the SM-BOSS trial, which compared 110 RYGB and 107 sleeve gastrectomy procedures, the authors observed no difference between RYGB and sleeve gastrectomy at five years with respect to weight loss, glycemic control, or complications requiring intervention. Of note, remission of acid reflux was observed in 60.4% of patients after RYGB compared with 25.0% after sleeve gastrectomy (P=0.002). Increasing acid reflux symptoms or escalation in reflux treatment also occurred more often after sleeve gastrectomy (31.8%) than after RYGB (6.3%) (P=0.006). 41 The SLEEVEPASS five year trial compared outcomes of 119 RYGB and 121 sleeve gastrectomy procedures and showed no significant differences in weight loss, remission of type 2 diabetes, or complications at five years 43 ; however at 10 years’ follow-up the percentage total weight loss was 3.5% higher for RYGB than sleeve gastrectomy (26.9% v 23.4%; P<0.001), but with no difference in type 2 diabetes remission rates. 46 An analysis that merged five year data from the SLEEVEPASS and SM-BOSS found that percentage total weight loss was 3.2% (95% confidence interval 1.6% to 4.7%) larger with RYGB than with sleeve gastrectomy, with no difference in type 2 diabetes remission between the two procedures and more complications in the RYGB cohort. 42 These findings are consistent with the multicenter US PCORnet Bariatric Study, an observational comparative effectiveness study of 24 982 patients undergoing RYGB and 18 961 patients undergoing sleeve gastrectomy, which showed significantly greater percentage total weight loss with RYGB than sleeve gastrectomy at five years (mean difference 6.7%, 5.8% to 7.7%), 48 a 10% higher rate of type 2 diabetes remission with RYGB than sleeve gastrectomy (hazard ratio 1.10, 1.04 to 1.16), a 25% lower type 2 diabetes relapse rate with RYGB than sleeve gastrectomy (hazard ratio 0.75, 0.67 to 0.84), 49 and a significantly lower risk of operation or intervention after sleeve gastrectomy than RYGB (hazard ratio 0.72, 0.65 to 0.79). 50
When comparing long term outcomes in bariatric surgery, considering observed differences in reoperation/reintervention rates and the durability of weight loss achieved with the various surgical approaches is important. In the SM-BOSS trial, reoperation or reintervention was reported in 15.8% (16/101) of patients after sleeve gastrectomy and 22.1% (23/104) after RYGB. The most frequent indications for reintervention were acid reflux for sleeve gastrectomy and internal hernia for RYGB. 41 In the 10 year SLEEVEPASS paper, the authors reported reoperation rates of 15.7% and 18.5% for sleeve gastrectomy and RYGB, respectively. 46 When considering differences in weight regain between the procedures, the PCORnet Bariatric Study found that weight regain to within 5% of the preoperative baseline occurred least often among patients who had RYGB (3.3%), followed by those who had sleeve gastrectomy (12.5%) and AGB (36.0%), at five year follow-up. These findings have been corroborated by two other smaller retrospective observational studies. 51 52 Collectively, these studies highlight the important trade-offs between the benefits and risks of RYGB and sleeve gastrectomy, to help to inform shared decision making conversations with patients ( table 4 ).
Evidence summary comparing outcomes of Roux-en-y gastric bypass and sleeve gastrectomy to inform shared decision making
OAGB is surgical procedure that uses a long gastric pouch connected by a single wide gastro-jejunal anastomosis to a loop of jejunum 150-200 cm distal to the ligament of Treitz, thus creating a gastric bypass by way of a loop and with a single anastomotic connection ( fig 2 ). 65 66 In a meta-analysis of 25 RCTs comparing OAGB and RYGB, including a total of 2715 patients, RYGB showed a better weight loss after three months (two studies, 131 patients; mean difference 2.41%, 0.46% to 4.36%; I 2 =76%, P=0.02), six months (two studies, 69 patients; 3.83%, 2.46% to 5.21%; I 2 =5%, P<0.001), one year (three studies, 180 patients; 6.35%, 4.69% to 8.01%; I 2 =0%, P<0.001), and five years (two studies, 128 patients; 3.90%, 1.21% to 6.59%; I 2 =0%, P=0.005). 67 In terms of remission of type 2 diabetes, two RCTs have been published but no meta-analysis has been done. 66 68 In the first study, among 33 patients randomized only three had type 2 diabetes—one in the OAGB group and two in the RYGB group—all of whom had remission at five years. 68 In a French study, 253 patients were randomized to RYGB or OAGB, but no significant difference was found between the type 2 diabetes remission rates at two years. 66 An International Federation for the Surgery of Obesity (IFSO) position statement on OAGB reviewed 95 studies with a total of 23 341 patients and found limited data on complications reported from seven out of 95 studies of OAGB. Perioperative complications occurred in 5.5% and reoperation in 1.0%, and perioperative mortality was low (<0.05%). Late complications occurred in 5.5%, including marginal ulcers, bowel obstruction, protein malnutrition, and biliary reflux, with a reoperation rate of 1.3%. 65

One anastomosis gastric bypass. Adapted from ObesityGoAway. Mini gastric bypass or one anastomosis gastric bypass (OAGB) ( obesitygoaway.com/services )
Observational studies have compared longer term effects of gastric bypass and sleeve gastrectomy with respect to established cardiovascular risk factors, such as hypertension and dyslipidemia. 53 54 69 In the ENGAGE CVD study, patients from nine practices having RYGB and sleeve gastrectomy (n=4964) were compared over five years to assess remission and relapse rates for hypertension. After five years, without accounting for relapse, 42% of patients having RYGB and 43% of those having sleeve gastrectomy experienced remission of hypertension. When accounting for relapse, 17% of patients having RYGB and 18% of those having sleeve gastrectomy remained in remission at five years, with no statistical differences noted between procedures. 53 Another study similarly compared the effect of RYGB and sleeve gastrectomy on dyslipidemia with up to four years of follow-up. Without accounting for relapse, remission of dyslipidemia was achieved in 58.9% of patients after RYGB and 51.9% following sleeve gastrectomy after four years. 54 After accounting for relapse, remission of dyslipidemia was still significantly higher after RYGB (38.0%) compared with sleeve gastrectomy (28.0%), after four years. Regarding changes in predicted cardiovascular risk, a study using the ENGAGE CVD data found no significant difference between RYGB and sleeve gastrectomy in changes in predicted 10 year risk of atherosclerotic cardiovascular disease five years after surgery. 69
Risks of metabolic/bariatric surgery
The perioperative risks of MBS have declined in the laparoscopic era. 70 Perioperative mortality is between 0.1% and 1.1%, and perioperative morbidity varies widely between 2% and 20% depending on both the specific type of procedure and characteristics of patients. 71 72 In the longer term, from a large, national comparative outcomes study of MBS procedures at five years, operation or intervention, endoscopy, and hospital admission were more likely after RYGB than after sleeve gastrectomy, but no difference in mortality was seen. 50
In the past decade, more studies have assessed the potential for non-operative adverse outcomes following MBS, including the risk of substance and alcohol use disorders and suicide or accidental deaths. 73 Overall, 18 observational studies with sample sizes ranging from 50 patients to >4000 patients, indicate that MBS is associated with an increased risk of alcohol and substance use disorders compared with usual care. 74 75 A meta-analysis of five observational studies at three years after surgery found that the pooled odds of alcohol use disorder were 1.83 (1.53 to 2.18; P<0.001) for RYGB compared with non-surgical treatment. In a matched controlled, multisite study of US veterans (predominantly men), eight years after a sleeve gastrectomy, the probability of unhealthy alcohol use was higher in surgical versus control patients (7.9% (95% confidence interval 6.4% to 9.5%) versus 4.5% (4.1% to 4.9%); difference 3.4% (1.8% to 5.0%)). Similarly, eight years after an RYGB, the probability of unhealthy alcohol use was higher in surgical than control patients (9.2% (8.0% to 10.3%) versus 4.4% (4.1% to 4.6%); difference 4.8% (3.6% to 5.9%)). 62 Some mechanisms have been proposed to explain these findings, including pharmacokinetic studies showing higher peak blood alcohol concentrations after RYGB compared with controls, changes in reward sensitivity via a neurobiological mechanism, changes to the ghrelin system, and altered genetic expression in some regions of the brain. 76 Together, these findings strongly suggest that education, screening, evaluation, and referral for treatment should be incorporated into both preoperative and postoperative bariatric surgical care as well as into careful lifelong monitoring in primary care settings.
An early study in the US showed that suicide rates in one state over 10 years among post-MBS patients were 13.7 per 10 000 among men and 5.2 per 10 000 among women, rates much higher than in age and sex matched US controls (2.4/10 000 men aged 35-64; 0.7/10 000 women aged 35-64). 77 A systematic review including 28 studies estimated an overall suicide rate of 4.1 per 10 000 person years, which was higher than in the general population. 78 Not included in this review, a more recent Canadian study examined self-harm emergencies both before and after MBS and found that the rate of these events increased from 2.3 events per 1000 people three years before surgery to 3.6 events per 1000 three years after surgery, with the most common cause being medication overdose. 79 A recent multisite study of US veterans involving more than 3800 patients having MBS and 34 000 carefully matched (including for mental health conditions) non-surgical controls with a mean follow-up of 4.6 years found that the risk of suicidal ideation was still significantly higher for post-MBS patients (adjusted hazard ratio 1.21, 1.03 to 1.41), as was risk of suicide attempt (1.62, 1.22 to 2.15). 64 On the other hand, another study of 12 000 cases of MBS from Western Australia showed no increased incidence of suicide or self-harm in the MBS population after an average of three years’ follow up. 80 Finally, a recent Swedish registry study of more than 22 500 people showed that an increased risk of self-harm diagnoses, hospital admissions for depression, and completed suicides two years after MBS was completely attributed to a previous history of self-harm or depression that was present before the MBS procedure. 81 These authors note that bariatric patients may be a particularly vulnerable population that could benefit from preoperative screening and recognition of these problems before surgery. In addition, the US American Society for Metabolic and Bariatric Surgery (ASMBS) issued a position statement on preoperative optimization before surgery that includes guidance to identify, treat, and optimize any existing psychiatric symptoms. 82 These and other data in the literature clearly show signs of increased risk around harmful behaviors in patients after MBS, and the question remaining is how to best identify who, how, and why this occurs.
Metabolic/bariatric surgery and cancer
Obesity is associated with an increased risk of developing certain cancers. 83 Observational data have been published with respect to cancer (risk) outcomes after MBS, mostly showing a reduction in both obesity related and all cause cancer cases and cancer related mortality. A recent systematic review identified eight observational studies including more than 600 000 patients and found that MBS was associated with a reduced risk of all types of cancer (pooled odds ratio 0.72, 0.59 to 0.87) and of obesity associated cancer (0.55, 0.31 to 0.96). 84 A single large, multisite cohort study that compared 22 198 patients with severe obesity who underwent MBS and 66 427 non-surgical controls showed a 33% lower risk of incident cancer of any type (hazard ratio 0.67, 0.60 to 0.74; 488 incident cases in MBS group over 87 071 person years versus 2055 incident cases in non-surgical group over 228 010 person years) and a larger reduction in obesity associated cancers, such as postmenopausal breast cancer (0.58, 0.44 to 0.77) and endometrial cancer (0.50, 0.37 to 0.67). 85 In a recently published retrospective cohort study of more than 30 000 patients, MBS (RYGB and sleeve gastrectomy) was significantly associated with a lower risk of obesity associated cancer (adjusted hazard ratio 0.68, 0.53 to 0.87) and cancer related mortality (0.52, 0.31 to 0.88). 86 However, for incident colorectal cancer, some studies have reported an increased risk with MBS, whereas others report a decreased risk. In a systematic review of 18 studies and more than 12 million patients, MBS was found to be significantly protective for colorectal cancer incidence (hazard ratio 0.81; P=0.0142). The protective effect persisted for subgroups of women (relative risk 0.54; P=0.0014) but not for men (0.74; P=0.2798). No differences were found between surgical procedures. 87
Obesity may accelerate and promote cancer growth by multiple potential mechanisms, including increased circulating estrogens, inflammatory cytokines, circulating adipokines, insulin, and insulin growth factor; higher cell proliferation; changes in microbiota; and epigenetic changes. Better elucidation of the specific biological mechanism(s) of effect in humans that are responsible for the observed changes in cancer risk with MBS is needed, as is identification of valid biomarkers to predict these clinical outcomes. The main concern with drawing definitive conclusions from any of the observational studies is the potential role of unmeasured confounding and selection bias, and a large, randomized trial is needed to further validate this association.
Obesity and metabolic/bariatric surgery in the era of covid-19
Several established risk factors for poor outcomes from covid-19 exist, perhaps none more initially apparent than obesity. At the outset of the covid-19 pandemic, data began to emerge that identified obesity as a predictor for greater need for hospital admission, intensive care, and mechanical ventilation, as well as mortality, after covid-19. 88 89 Obesity is associated with severe infection from respiratory viruses in general, and other obesity associated health conditions, such as type 2 diabetes and heart disease, can also increase this risk. Other hypotheses have included the effect of obesity on pulmonary function and perfusion, immune response, endocrine function, thromboembolic risk, and propagation by the pro-inflammatory obesity state of the hyperinflammatory response observed in severe covid-19. 90
As the pandemic evolved, observational data began to suggest a protective effect of previous MBS in people with covid-19. 91 92 93 94 95 A retrospective cohort study compared patients with obesity admitted with covid-19 who had had previous MBS (n=2607) versus those who had not (n=122 092). Patients with previous surgery had lower mortality (7.8% v 11.2%; P<0.0001) and intubation rates (18.5% v 23.6%; P<0.001) while in hospital compared with patients who had not had MBS. 91 A single site cohort study compared data from patients with previous MBS (n=11 809) matched 1:3 to patients with obesity without previous MBS. Test positivity rates were similar between the two groups; however, MBS was associated with significantly lower need for hospital admission and supplemental oxygen and less severe disease course of covid-19. 92 Three systematic reviews and meta-analyses have also been published. 93 94 95 The largest of these included nine articles and 1 130 341 people. It reported significantly reduced hospital admission (odds ratio 0.44, 0.45 to 0.61), need for intensive care (0.44, 0.29 to 0.67), and mortality (0.42, 0.25 to 0.70). 93
The covid-19 pandemic has highlighted another potential beneficial effect of MBS and introduced a debate about where MBS fits in the spectrum of medical necessity—should MBS be considered “essential” or “non-emergent” care? In addition, what has emerged from the pandemic are several useful consensus statements and practical delivery of care models for the surgical treatment of obesity during periods of resource strain or future pandemics. 22 96 97 98 99 Consistent across the consensus documents is an overarching opinion that patients with obesity and related conditions suffer when lifesaving therapies are delayed. In times of societal crisis, these patients should be prioritized for the full spectrum of obesity management therapies when possible; however, this must be balanced against local resource constraints and variables such as the perioperative cardiopulmonary risk of individuals when crisis related conditions are poor. 22 96 97 98 99
Shared decision making
The decision to undergo MBS should involve a shared decision making (SDM) process that considers the risks, benefits, and uncertainties of the operation. Ideally, this process should include clear communication from the surgeon, careful consideration of the patient’s values and preferences, and use of a decision aid for patients that provides objective information about all relevant treatment options and promotes involvement of patients in the decision making process. Many barriers to a high quality shared decision making process around MBS exist. 100 Recently, two US health systems identified six barriers as the most important to tackle: lack of insurance coverage, lack of patients’ and providers’ understanding of insurance coverage, lack of organizational prioritization of SDM, lack of knowledge about MBS, lack of interdepartmental clarity between primary care and specialty care around who should do what in terms of SDM, and limited training on SDM conversations and tools.
SDM around MBS generally occurs in two parts: a conversation with primary care or a non-surgical medical specialist about options for managing obesity and obesity related diseases to help patients to decide whether they are interested in a referral to learn more about their options; and a conversation with a metabolic/bariatric surgeon and other members of a multidisciplinary bariatric team to discuss the benefits and risks of alternative approaches in greater detail and ensure alignment of these treatments with the patient’s preferences. 101 A typical SDM workflow for MBS includes conducting a comprehensive assessment of the patient’s medical history, current health status, and overall health and wellbeing to determine whether the patient is a candidate for MBS; sharing educational materials and resources, such as information about the surgery and recovery process, potential side effects, and long term risks and benefits, to help the patient to better understand the treatment options and make a more informed decision; discussion of options including their long term risks, benefits, and uncertainties ( table 4 summarizes these for the two most common operations) and tailoring of information to the individual patient’s demographic (for example, race/ethnicity 102 ) and clinical situation using risk calculators, 103 104 105 where possible; and clarification of the patient’s goals and expectations for treatments, as well as their own values and preferences around the risks and benefits of the different approaches.
To date, only one randomized trial has evaluated a decision aid for patients considering MBS, 106 involving 152 patients. In this study, a video based decision aid seemed to result in a greater improvement than an educational booklet in terms of patients’ knowledge, decisional conflict, and outcome expectancies, but it did not have a significant effect on patients’ decisions to undergo surgery. Several other decision aids have been developed, but few have been rigorously tested. 107 108 109 110 A recent systematic review found that the quality of online information for patients about MBS is generally poor and does not meet international standards for decision making. Other more rigorously developed decision aids generally support the one randomized trial in showing that decisional conflict can be decreased and use of SDM with a decision aid could influence the choice of operation. Other risk calculators have been developed to help to provide patient specific estimates of the benefits and risks of MBS. 103 104 111
Emerging bariatric procedures/treatments
A new field of endoscopic bariatric procedures (EBPs) has evolved in the past several years for use in people who fail to lose weight with lifestyle or drug therapies and for patients not eligible for or not interested in an MBS procedure ( fig 3 ). The purported advantages are that these are less invasive, less costly, reversible, and repeatable. EBPs have been developed that are either gastric interventions or small bowel interventions. 112 113 Intragastric balloons function by taking up space in the stomach, creating a sense of fullness and delaying gastric emptying. They are generally indicated for a lower BMI threshold of 30-40 and result in modest, temporary weight loss. Several different manufacturers and types of intragastric balloons exist—single or multiple, filled with saline or gas, placed by endoscopy or swallowed in a capsule—some of which have a recommended four to 12 month limited implantation time and can be used as repeated treatments. Seven RCTs in more than 1000 people show that intragastric balloons result in significant weight reduction compared with control groups, with 10-15% total body weight loss at the time of removal of the balloon (six months) and 6-8% at 12 months. 114 115 The most common complications include nausea, vomiting, and abdominal pain in more than 20% of people, especially in the first weeks after placement when adaptation to the device is taking place. More serious complications such as obstruction, perforation, or death can occur; these are rare, but patients must be monitored closely for signs of balloon deflation or obstruction. In 2017 the US Food and Drug Administration (FDA) issued a warning about two newly recognized complications of fluid filled balloons—balloon hyperinflation and balloon related pancreatitis—for which patients should be carefully monitored and early removal of the device is indicated in an FDA letter. 116

Emerging endoluminal metabolic/bariatric procedures. A: intragastric balloon (adapted from Intragastric balloon - Mayo Clinic); B: transpyloric shuttle (adapted from Transpyloric shuttle (TPS) ( researchgate.net )); C: primary obesity surgery endoluminal (adapted from POSE ( researchgate.net )); D: endoscopic sleeve gastroplasty (adapted from ESG. The pressure is on! Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: toward better patient allocation beyond pygmalionism - Gastrointestinal Endoscopy ( giejournal.org )); E: duodenal liner (adapted from Duodenal liner Various Novel and Emerging Technologies in Endoscopic Bariatric and Metabolic Treatments ( e-ce.org ))
A transpyloric shuttle is a device that consists of a large silicone ball that is connected to a smaller one via a tether so that when food is ingested peristalsis moves the larger ball on to the pylorus, causing a temporary obstruction that delays gastric emptying. The transpyloric shuttle typically dwells in the stomach for 12 months. In two studies, weight loss results were similar to those with intragastric balloons. 117 118 In the pivotal randomized trial for US approval, 270 patients were randomized and the transpyloric shuttle group had 9.3% initial weight loss compared with 2.8% in sham controls at 12 months; 67% of patients with a transpyloric shuttle achieved at least 5% initial weight loss at 12 months. 117 Primary obesity surgery endoluminal (POSE) uses an endoscopic approach to create full thickness gastric tissue plications (folds) along the fundus and distal body of the stomach. In one 12 month observational study of 147 patients and in two RCTs of 43 and 332 patients each, total weight loss ranged from 5% to 15% for the POSE device group. 119 Endoscopic sleeve gastroplasty (ESG) is a minimally invasive endoscopic procedure that uses a full thickness suturing system to create a sleeve shaped stomach. No randomized trials have been conducted, but case series have shown weight loss of 17-19% at 12 months and improvements in type 2 diabetes and hypertriglyceridemia. 112 120 A meta-analysis of 1772 patients showed weight loss of 15% at six months, 16% at 12 months, and 17% at 24 months, with a severe adverse event rate of 2%. 121 Compared with surgical sleeve gastrectomy in a 137 patient case matched study, ESG led to significantly less weight loss but fewer adverse events. 122
Small bowel interventions include a duodenal-jejunal bypass liner that functions as a malabsorptive device preventing contact of food substances with the intestinal mucosa. It anchors from the duodenal bulb and extends to the proximal jejunum and dwells there for 12 months. Longer term results are lacking, and six month case series data show modest weight loss (6-7 kg in 19 people with BMI 34-38) and conflicting results on diabetes outcomes. 123 A randomized trial including more than 300 people in the US was stopped owing to higher than anticipated rates of hepatic abscess. Duodenal mucosal resurfacing is another technique that uses a catheter to heat ablate the duodenal mucosa, which may result in improved glycemic control. A small case series study involving 39 patients showed glycated hemoglobin to be improved by 1.4% or 0.7% at six months depending on long or short ablation. 124 Interestingly, no correlation was seen between magnitude of weight loss and glycemic improvement. An international multicenter case series study examined duodenal mucosal resurfacing in 46 patients with type 2 diabetes with and without fatty liver disease and showed a 0.9% reduction in glycated hemoglobin and improvement in liver enzymes at 12 months. 125 126 For all these EBPs, additional studies are needed on long term efficacy, outcomes of metabolic diseases, and comparisons with best available drug therapy and bariatric surgical options.
Considering the substantial advances in the understanding of the disease of obesity, its management, and MBS specifically, the leaderships of the ASMBS and IFSO recently produced a joint statement summarizing the literature and updating the recommended indications for MBS. 127 These include consideration of MBS in people with lower BMIs (BMI 30-35 with metabolic disease (including type 2 diabetes) if non-surgical therapy is ineffective; BMI 35-40 with or without comorbid conditions), in people with different ethnic backgrounds (Asians with BMI >27.5), in younger and older patients, and as a bridge to other medically necessary procedures such as joint replacement or organ transplantation. The recommendation for metabolic disease treatment with MBS at lower BMI is consistent with and supported by a previous statement from international diabetes organizations from the second Diabetes Surgery Summit (DSS-II) in 2016, which summarized the large body of data from RCTs in people with class 1 obesity and type 2 diabetes. 21
These guidelines now go beyond those from the National Institute for Health and Care Excellence (NICE) in the UK, which state that bariatric surgery is a recommended treatment option for people with a BMI of 40 or more or between 35 and 40 with other significant disease (for example, type 2 diabetes or high blood pressure) that could be improved if they lost weight. 128 However, the NICE and ASMBS/IFSO guidelines are consistent for adults with recent onset of type 2 diabetes whose diagnosis has been made within the previous 10 years, as NICE recommends offering an expedited assessment for bariatric surgery to people with a BMI of 35 or over, for those with a BMI of 30 to 34.9, and for people of South Asian, Chinese, other Asian, Middle Eastern, Black African, or African-Caribbean family background with a BMI of 27.5 and over. Although the US Preventive Services Task Force recommends that clinicians offer or refer adults with a BMI of 30 or higher for intensive, multicomponent behavioral interventions (B recommendation), it does not provide any recommendations on MBS. 129
Conclusions
The escalating rates of obesity and type 2 diabetes have increased interest in MBS to reduce the long term complications of these diseases. The evidence base for MBS has grown substantially over recent decades, but further research is urgently needed to fill major gaps and inform clinical decision making. Recent five year and 10 year randomized trials provide strong evidence supporting the long term effectiveness and safety of sleeve gastrectomy, RYGB, and biliopancreatic diversion. MBS has a lasting positive impact on weight loss and comorbidity improvement, especially in patients with type 2 diabetes. However, different procedures have trade-offs, with RYGB and biliopancreatic diversion offering the greatest improvement but carrying higher risks.
A major challenge to the field is the lack of a large, long term trial comparing MBS with best available medical treatment for obesity, particularly given the influx of new anti-obesity drugs, which now rival some MBS operations in terms of weight loss outcomes. We believe that the long term path forward for many patients with severe obesity will not be an either/or choice with regard to MBS and anti-obesity drugs but will be more of an adjuvant therapeutic approach. Finally, given the potential for some negative medical and psychological consequences of MBS, long term follow-up and psychosocial support are crucial. Primary care providers can help to mitigate potential harms of MBS by encouraging appropriate follow-up and screenings ( box 1 ). 130
Recommended postoperative screening and follow-up
Monitor progress with weight loss and weight regain; consider evaluation by bariatric medicine provider, nutrition/medications if regain occurs
Avoid non-steroidal anti-inflammatory drugs
Consider gout and gallstone prophylaxis
Screen annually for depression and alcohol and substance use disorders; refer for treatment as needed
Encourage long term daily bariatric formulation vitamin supplementation to reduce risk of nutritional deficiencies
Annual nutritional monitoring, 24 hour urine calcium excretion (for biliopancreatic diversion), vitamin B 12 , folic acid, iron studies, 25-hydroxy vitamin D, intact parathyroid hormone
Bone density screening (dual x ray absorptiometry) every two years
Glossary of abbreviations
AGB—adjustable gastric banding
ASMBS—American Society for Metabolic and Bariatric Surgery
BMI—body mass index
EBPs—endoscopic bariatric procedures
ESG—endoscopic sleeve gastroplasty
FDA—Food and Drug Administration
IFSO—International Federation for the Surgery of Obesity
MACE—major adverse cardiovascular events
MASH—metabolic dysfunction associated steatohepatitis
MASLD—metabolic dysfunction associated steatotic liver disease
MBS—metabolic/bariatric surgery
NICE—National Institute for Health and Care Excellence
OAGB—one anastomosis gastric bypass
POSE—primary obesity surgery endoluminal
RCT—randomized controlled trial
RYGB—Roux-en-y gastric bypass
SDM—shared decision making
Questions for future research
Is metabolic/bariatric surgery more effective than emerging drug therapy for long term treatment of obesity and type 2 diabetes?
Is the long term (≥10 year) effect of sleeve gastrectomy on weight loss and comorbidity improvement as durable as that of gastric bypass?
What is the relative effect of sleeve gastrectomy and gastric bypass on long term rates of reoperation, hospital admission, and healthcare cost?
What patient level factors can predict success (weight loss, health improvements, and cost savings) after bariatric surgical procedures?
What are the mechanisms of effect for the increase in self-harming behaviors and the reduction in cancer risk after metabolic/bariatric surgery?
What clinical role do emerging metabolic/bariatric procedures have relative to the two most common, sleeve gastrectomy and gastric bypass? How do they compare for long term efficacy and safety?
How patients were involved in creation of this article
After email communication from the authors, one of our long time bariatric patient research partners agreed to review and provide feedback on our manuscript outline, plan, and final drafts
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: All authors contributed equally to the planning, conduct, and reporting of the work described in the article and meet all four ICJME criteria for authorship. APC and DEA are the guarantors.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: APC has received a research grant from Allurion Technologies.
Provenance and peer review: Commissioned; externally peer reviewed.
- Arterburn DE ,
- Courcoulas AP
- ↵ Centers for Disease Contro. and Prevention. Adult Obesity Facts. 2022. https://www.cdc.gov/obesity/data/adult.html#print .
- ↵ NHS Digital. Health Survey for England. 2017. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2017 .
- ↵ Eurostat. Overweight and Obesity - BMI Statistics. 2021. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Overweight_and_obesity_-_BMI_statistics#Obesity_by_age_group .
- Williamson K ,
- Nimegeer A ,
- ↵ American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011-2020. 2022. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers .
- Welbourn R ,
- Hollyman M ,
- Kinsman R ,
- Halperin F ,
- Simonson DC ,
- Wewalka M ,
- Cummings DE ,
- Westbrook EO ,
- O’Brien PE ,
- Playfair J ,
- Wentworth JM ,
- Goldfine AB
- Kirwan JP ,
- Courcoulas AP ,
- Schauer PR ,
- STAMPEDE Investigators
- Ikramuddin S ,
- Gallagher JW ,
- Neiberg RH ,
- Mingrone G ,
- Panunzi S ,
- De Gaetano A ,
- Nathan DM ,
- Delegates of the 2nd Diabetes Surgery Summit
- Pouwels S ,
- Aggarwal S ,
- Aminian A ,
- Al-Kurd A ,
- Verrastro O ,
- Castagneto-Gissey L ,
- Albaugh VL ,
- Kindel TL ,
- Nissen SE ,
- Sjöström L ,
- Peltonen M ,
- Jacobson P ,
- Fisher DP ,
- Johnson E ,
- Haneuse S ,
- Zajichek A ,
- Stenberg E ,
- Sundbom M ,
- Jernberg T ,
- Ardissino M ,
- Näslund E ,
- Hofmann R ,
- Doumouras AG ,
- Paterson JM ,
- Mentias A ,
- Youssef D ,
- VanderWeele TJ ,
- Arterburn D
- Arterburn D ,
- Schauer P ,
- Pereira TV ,
- Billeter AT ,
- Scheurlen KM ,
- Peterli R ,
- Wölnerhanssen BK ,
- Salminen P ,
- Grönroos S ,
- Kashyap SR ,
- Wellman R ,
- Emiliano A ,
- PCORnet Bariatric Study Collaborative
- McTigue KM ,
- Courcoulas A ,
- Coleman KJ ,
- Maciejewski ML ,
- Van Scoyoc L ,
- Reynolds K ,
- Barton LJ ,
- Campbell JA ,
- El Badaoui J ,
- Berkowitz TSZ ,
- De Luca M ,
- Espalieu P ,
- Pelascini E ,
- Douissard J ,
- Podetta M ,
- Piñango S ,
- Fischer H ,
- Campos GM ,
- Khoraki J ,
- Browning MG ,
- Pessoa BM ,
- Mazzini GS ,
- Birkmeyer NJ ,
- Dimick JB ,
- Michigan Bariatric Surgery Collaborative
- Backman O ,
- Stockeld D ,
- Rasmussen F ,
- Shahrestani S ,
- Verhoeff K ,
- Steffen KJ ,
- Wonderlich JA ,
- Pollert GA ,
- Tindle HA ,
- Hammers J ,
- Peterhänsel C ,
- Petroff D ,
- Klinitzke G ,
- Kersting A ,
- Bhatti JA ,
- Nathens AB ,
- Thiruchelvam D ,
- Grantcharov T ,
- Goldstein BI ,
- Redelmeier DA
- Morgan DJ ,
- Lagerros YT ,
- Hedberg J ,
- Birriel TJ ,
- Avgerinos KI ,
- Mantzoros CS ,
- Wiggins T ,
- Antonowicz SS ,
- Schauer DP ,
- Feigelson HS ,
- Koebnick C ,
- Chierici A ,
- Amoretti P ,
- Simonnet A ,
- Chetboun M ,
- LICORN and the Lille COVID-19 and Obesity study group
- Hohmann SF ,
- Milinovich A ,
- Wolski KE ,
- Kattan MW ,
- Hasnain N ,
- Farhan SA ,
- Executive Council of ASMBS
- Shikora S ,
- Daigle CR ,
- Augustin T ,
- Sarwer DB ,
- Gasoyan H ,
- Bauerle Bass S ,
- Spitzer JC ,
- Fitzpatrick SL ,
- ↵ Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. Bariatric Surgical Risk/Benefit Calculator. https://riskcalculator.facs.org/bariatric/ .
- ↵ Michigan Bariatric Surgery Collaborativ. Weigh the Odds, MBSC Outcomes Calculator https://www.michiganbsc.org/DecisionTools/ .
- Brethauer SA ,
- Andalib A ,
- Bogart TA ,
- Sepucha KR ,
- Musbahi A ,
- Viswanath YKS ,
- Gopinath BR
- Nijland LMG ,
- Noordman PCW ,
- van Veen RN ,
- Bonjer HJ ,
- de Castro SMM
- Varban OA ,
- Bonham AJ ,
- Stricklen AL ,
- Sharaiha RZ
- Abu Dayyeh BK ,
- Muniraj T ,
- Teigen LM ,
- ↵ US Food and Drug Administration. The FDA alerts health care providers about potential risks with liquid-filled intragastric balloons. 2017. https://www.fda.gov/medical-devices/letters-health-care-providers/fda-alerts-health-care-providers-about-potential-risks-liquid-filled-intragastric-balloons .
- ↵ BAROnova. Transpyloric Shuttle/Transpyloric Shuttle Delivery Device Instructions for Use. 2015. https://www.accessdata.fda.gov/cdrh_docs/pdf18/P180024D.pdf .
- López-Nava G ,
- Bautista-Castaño I ,
- Jimenez A ,
- de Grado T ,
- Fernandez-Corbelle JP
- Sharaiha RZ ,
- Hedjoudje A ,
- Cheskin LJ ,
- Schweitzer M ,
- Federspiel CA ,
- Vilmann P ,
- Rajagopalan H ,
- Cherrington AD ,
- Thompson CC ,
- van Baar ACG ,
- Holleman F ,
- Crenier L ,
- ↵ Fractyl Health. Fractyl Announces Revita-2 Data Showing Revita DMR Provides Statistically Significant Improvements in Blood Glucose and Liver Fat in Type 2 Diabetes with and without NAFLD. 2019. https://www.fractyl.com/fractyl-announces-revita-2-data-showing-revita-dmr-provides-statistically-significant-improvements-in-blood-glucose-and-liver-fat-in-type-2-diabetes-with-and-without-nafld/ .
- Eisenberg D ,
- Shikora SA ,
- ↵ National Institute for Health and Care Excellence. Obesity: identification, assessment and management: Surgical Interventions. 2022. https://www.nice.org.uk/guidance/cg189/chapter/Recommendations#surgical-interventions .
- ↵ US Preventive Services Task Force. Weight Loss to Prevent Obesity-Related Morbidity and Mortality in Adults: Behavioral Interventions. Final Recommendation Statement. 2018. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/obesity-in-adults-interventions .
- Mechanick JI ,
- Apovian C ,
- Brethauer S ,
- Introduction
- Conclusions
- Article Information
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); HCC, hepatocellular carcinoma; RYGB; roux-en-Y gastric bypass; and SG, sleeve gastrectomy.
Composite cardiovascular events were defined as the first occurrence of unstable angina, myocardial infarction, or revascularization, including percutaneous coronary intervention or coronary artery bypass graft; composite end point of cerebrovascular events, defined the first occurrence of stroke (ischemic or hemorrhagic stroke), cerebral infarction, transient ischemic attack, carotid intervention, or surgery; composite coronary artery interventions, based on the requirement for coronary stenting, percutaneous coronary intervention, or coronary artery bypass.
a Statistically significant difference.
eAppendix. Supplementary Methods
eTable 1. Sensitivity Analysis Measuring Outcomes Associated With Bariatric Surgery vs No Bariatric Surgery in Patients With Obesity and Nonalcoholic Fatty Liver Disease After Excluding Index Events Within 2 Years After the Index Date
eTable 2. Secondary Analysis Measuring Outcomes From the Initial Time of Diagnosis of Nonalcoholic Fatty Liver Disease as the Index Event
eTable 3. Postoperative Complications Within 30 Days After Bariatric Surgery
eFigure 1. Propensity Score Density Graph for Patients With Nonalcoholic Fatty Liver Disease and Obesity Who Underwent Bariatric Surgery vs Controls Who Did Not Before and After Propensity Score Matching
eFigure 2. Kaplan-Meier Curve Compares Cardiovascular Diseases in Patients Who Underwent Bariatric Surgery vs Controls Who Did Not
eFigure 3. Kaplan-Meier Curve Compares Mortality in Patients Who Underwent Bariatric Surgery vs Control Who Did Not
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Krishnan A , Hadi Y , Alqahtani SA, et al. Cardiovascular Outcomes and Mortality After Bariatric Surgery in Patients With Nonalcoholic Fatty Liver Disease and Obesity. JAMA Netw Open. 2023;6(4):e237188. doi:10.1001/jamanetworkopen.2023.7188
Manage citations:
© 2024
- Permissions
Cardiovascular Outcomes and Mortality After Bariatric Surgery in Patients With Nonalcoholic Fatty Liver Disease and Obesity
- 1 Section of Gastroenterology and Hepatology, West Virginia University School of Medicine, Morgantown
- 2 Division of Gastroenterology and Hepatology, Johns Hopkins University School of Medicine, Baltimore, Maryland
- 3 West Virginia Clinical & Translational Science Institute, Morgantown
- 4 WVU Medicine Center for Weight Management, Morgantown, West Virginia
- 5 Department of Surgery, West Virginia University School of Medicine, Morgantown
Question Is bariatric surgery associated with the incidence of major adverse cardiovascular events or all-cause of mortality among patients with nonalcoholic fatty liver disease (NAFLD) and obesity?
Findings In this cohort study of 152 394 patients with NAFLD and obesity (including 4693 patients who underwent bariatric surgery and 147 701 patients in the nonsurgical control group), bariatric surgery was significantly associated with lower risks of major adverse cardiovascular events and all-cause mortality. These outcomes were consistent at follow-up durations of 1, 3, 5, and 7 years.
Meaning These findings suggest that bariatric surgery was associated with a substantially lower risk of incidents of major adverse cardiovascular events and all-cause mortality in patients with NAFLD and obesity.
Importance Bariatric surgery (BS) is associated with significantly reduced incidence of cardiovascular diseases and mortality in patients with obesity. However, whether BS can decrease major adverse cardiovascular events in patients with nonalcoholic fatty liver disease (NAFLD) remains poorly understood.
Objective To investigate the association of BS with the incidence of adverse cardiovascular events and all-cause mortality in patients with NAFLD and obesity.
Design, Setting, and Participants This was a large, population-based, retrospective cohort using data from the TriNetX platform. Adult patients with a body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) of 35 or greater and NAFLD (without cirrhosis) who underwent BS between January 1, 2005, and December 31, 2021, were included. Patients in the BS group were matched with patients who did not undergo surgery (non-BS group) according to age, demographics, comorbidities, and medication by using 1:1 propensity matching. Patient follow-up ended on August 31, 2022, and data were analyzed in September 2022.
Exposures Bariatric surgery vs nonsurgical care.
Main Outcomes and Measures The primary outcomes were defined as the first incidence of new-onset heart failure (HF), composite cardiovascular events (unstable angina, myocardial infarction, or revascularization, including percutaneous coronary intervention or coronary artery bypass graft), composite cerebrovascular disease (ischemic or hemorrhagic stroke, cerebral infarction, transient ischemic attack, carotid intervention, or surgery), and a composite of coronary artery procedures or surgeries (coronary stenting, percutaneous coronary intervention, or coronary artery bypass). Cox proportional hazards models were used to estimate hazard ratios (HRs).
Results Of 152 394 eligible adults, 4693 individuals underwent BS; 4687 patients who underwent BS (mean [SD] age, 44.8 [11.6] years; 3822 [81.5%] female) were matched with 4687 individuals (mean [SD] age, 44.7 [13.2] years; 3883 [82.8%] years) who did not undergo BS. The BS group had significantly lower risk of new-onset of HF (HR, 0.60; 95% CI, 0.51-0.70), cardiovascular events (HR, 0.53; 95% CI, 0.44-0.65), cerebrovascular events (HR, 0.59; 95% CI, 0.51-0.69), and coronary artery interventions (HR, 0.47; 95% CI, 0.35-0.63) compared with the non-BS group. Similarly, all-cause mortality was substantially lower in the BS group (HR, 0.56; 95% CI, 0.42-0.74). These outcomes were consistent at follow-up duration of 1, 3, 5, and 7 years.
Conclusions and Relevance These findings suggest that BS was significantly associated with lower risk of major adverse cardiovascular events and all-cause mortality in patients with NAFLD and obesity.
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of liver disorders in the US and is the leading cause of chronic liver disease globally. 1 The global prevalence of NAFLD has reached 25.2% and is projected to be 33.5% by 2030. 2 NAFLD is considered a hepatic manifestation of metabolic syndrome, which is characterized as a cluster of metabolic disorders, such as obesity, insulin resistance, type 2 diabetes, hypertriglyceridemia, dyslipidemia, and hypertension, which are all risk factors associated with cardiovascular diseases (CVDs). 3 Several cohort studies have steadily acknowledged that NAFLD is associated with a significantly higher risk of all-cause mortality and that the leading causes of death among patients with NAFLD are CVDs. 4 The multifaceted nature of NAFLD with varying coexisting metabolic complications makes its treatment complex. Interventions that target NAFLD-associated obesity could potentially reduce the incidence of CVDs and mortality in this patient group. However, no specific pharmacotherapy is approved to treat NAFLD, and lifestyle change aimed at weight loss remains the mainstay of clinical management. 4 , 5
Bariatric surgery (BS) is an efficient weight-loss intervention in patients with obesity. BS may be indicated in patients with obesity and NAFLD to achieve and maintain the degree of weight loss (ie, 7%-10%) associated with therapeutic outcomes. However, there is a paucity of data on the impact of BS specifically for patients with NAFLD, and the outcomes of BS in patients with obesity cannot be directly extrapolated for patients with NAFLD. The pathophysiology behind the association of NAFLD with CVD is not completely understood, and CVD development in NAFLD may involve other pathways besides obesity and insulin resistance alone, including low-grade inflammation, oxidative stress, and the effects of perturbations in the gut microbiota. 6 , 7 It has been known that patients with NAFLD constitute a higher-risk cohort for CVD independent of body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) and obesity. 7 Thus, the association of BS with NAFLD warrants further study. Therefore, we aimed to explore the association of BS with major adverse cardiovascular events and all-cause mortality at a population level.
This large, population-based, retrospective cohort study was conducted using TriNetX (Cambridge, Massachusetts), a federated health research network data set. TriNetX is a multi-institutional health research network that provides deidentified electronic health records (EHRs) from included health care organizations. Clinical variables are derived directly through EHRs. Robust quality assurance on the network is achieved at the time of extraction from the EHRs before inclusion in the data set. The platform only provides aggregate patient counts and statistical summaries to ensure deidentification at all levels of retrieval and dissemination of patient data. TriNetX received a waiver of review and informed consent from WIRB institutional review board as a federated network since it includes only aggregated counts and statistical summaries of deidentified information. Still, no protected health information was obtained, and no study-specific activities were performed in this retrospective analyses. Details of the data source are described in the eAppendix in Supplement 1 . We followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
All adult patients (aged >18 years) with a diagnosis of NAFLD and obesity with BMI of 35 or greater in the TriNetX database between January 1, 2005, and December 31, 2021, were identified. Follow-up of these patients ended on August 31, 2022. Patients were excluded if they met any of the following criteria: chronic liver disease other than NAFLD, including alcohol, viral, drug-induced, autoimmune, and genetic diseases, liver cirrhosis, or clinical diagnosis of hepatic decompensation (such as esophageal varices or ascites); BMI less than 35 (ineligible for bariatric surgery); history of excessive alcohol use, alcohol abuse, or alcohol use disorder or history of alcohol-related disorders; HIV infection; solid organ transplantation; and history of undergoing dialysis treatment. Finally, patients with a history of heart failure (HF), ischemic heart disease, unstable angina, myocardial infarction, aortic aneurysm or dissection, stroke (ischemic or hemorrhagic stroke), cerebral infarction, transient ischemic attack, carotid intervention or surgery, coronary stenting, percutaneous coronary intervention (PCI), or coronary artery bypass before inclusion to the cohort or prior to the index event were also excluded.
Patients who met the inclusion criteria were divided into 2 groups: those who underwent BS (study group) and those who did not undergo BS (control group). BS procedures included Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG). Patients with a history of gastric banding and other less common bariatric procedures were excluded. All bariatric surgical procedures were defined using the diagnosis and current procedural terminology suggested by the American Society for Metabolic and Bariatric Surgery for RYGB or SG. Details of diagnosis and procedure codes used for patient selection are described in the eAppendix in Supplement 1 .
BS was considered the index event for patients in the BS group. The index event for the control group was defined as the first time that the patient was eligible for inclusion in the study (based on a diagnosis of NAFLD and obesity with BMI ≥35) during the a priori–defined study time period (January 1, 2005, to December 31, 2021).
Each patient in the BS group was matched to a patient in the non-BS group using 1:1 propensity score matching (PSM) to reduce confounding. 8 Covariates in the propensity score model were adjusted for a priori–identified potential confounders: age, sex, self-reported race and ethnicity (Hispanic, non-Hispanic Black, non-Hispanic White, or non-Hispanic other [eg, Asian, American Indian or Alaska Native, Native Hawaiian, or other Pacific Islander]), nicotine dependence, BMI, diabetes, hypertension, hyperlipidemia, chronic respiratory disease, chronic kidney diseases, blood pressure, oral diabetes medication use, insulin use, use of angiotensin-converting enzyme inhibitor, β-blockers, antiarrhythmics, antilipemic agents, angiotensin receptor–blocker medications, use of any other antihypertensive medication, cholesterol level, low-density lipoprotein level, and serum triglyceride level. Race and ethnicity were included in analyses because of the associated differences in BS outcomes. 9 Logistic regression on these input matrices was used to obtain propensity scores for each patient in both cohorts. Logistic regression was performed in Python software version 3.6.5 (Python Software Foundation) using standard libraries NumPy and sklearn. The same analyses were also performed in R software version 3.4.4 (R Project for Statistical Computing) to ensure outputs matched. After calculating propensity scores, matching was performed using a greedy nearest-neighbor matching algorithm with a caliper of 0.1 pooled SDs. The order of the rows in the covariate matrix can affect the nearest neighbor matching; therefore, the order of the rows in the matrix was randomized to eliminate this bias.
The primary outcome of our study was to assess the incidence or new onset of major adverse cardiovascular events categorized as HF, composite cardiovascular events, composite cerebrovascular events, and composite coronary artery interventions. Composite cardiovascular events were defined as the first occurrence of unstable angina, myocardial infarction, or revascularization, including PCI or coronary artery bypass graft. 10 , 11 Composite of cerebrovascular disease was defined as the first occurrence of stroke (ischemic or hemorrhagic stroke), cerebral infarction, transient ischemic attack, carotid intervention, or surgical procedure. 10 , 11 The composite of coronary artery procedures or surgical treatments was based on the requirement for coronary stenting, PCI, or coronary artery bypass. The secondary outcome was to evaluate the incidence of all-cause mortality. Patients with CVD and mortality outcomes before the index period were excluded at the time of patient selection. Major surgical complications were estimated to assess the safety of BS in this patient population 30 days after BS.
All statistical analyses were performed in real time using the TriNetX platform. Continuous variables are expressed as mean and SD. Categorical variables are presented as frequency and percentage. Patients were matched using PSM, and balancing of potential confounding variables between the BS and the control group after PSM was evaluated using standardized mean differences (SMD) with a threshold set a priori at 0.10. SMD was used to measure the magnitude of difference between the groups rather than the P value because of the insensitivity of SMDs to sample size. 12 For each outcome, Cox proportional hazards models were used to estimate the hazard ratios (HRs). HRs and 95% CIs, along with tests for proportionality, were calculated using the survival package in R version 3.2.3. HRs and 95% CIs adjusted for baseline variables were calculated and reported for all analyses. Numbers were then validated by comparing them with the output from SAS statistical software version 9.4 (SAS Institute). Kaplan-Meier survival analyses were also used to estimate the survival probability of the outcome at the end of 7 years after the index event. Patients were censored when the time window ended or on the day after the last event in their record. Hypothesis testing for Kaplan-Meier survival curves was conducted by using the log-rank test. A priori 2-sided α < .05 was used for statistical significance. Data were analyzed in September 2022.
The benefits of BS may not be apparent immediately after the procedure, and any short-term outcomes in this population may be due to multiple chronic high-risk factors. Therefore, we performed a sensitivity analysis by estimating risk after excluding patients with outcomes within 1 year or 2 years after the index event.
Secondary analysis was performed comparing CVD outcomes between a cohort of patients with obesity without NAFLD who underwent BS and patients with obesity without NAFLD who did not undergo BS. HRs were calculated, adjusted for the same confounders as the primary analyses.
A total of 152 394 patients with obesity and NAFLD were identified. Among these patients, 4693 had a BS history (BS group), and 147 701 did not have a BS history. Of the BS group, SG accounted for 65% of procedures, whereas the rest had a history of RYGB. ( Figure 1 ). After PSM, a total of 4687 patients were included in each group (BS: mean [SD] age, 44.8 [11.6] years; 3822 [81.5%] female; control: mean [SD] age, 44.7 [13.2] years; 3883 [82.8%] female). The groups were well-matched after PSM (SMD, <0.1) ( Table 1 ; eFigure 1 in Supplement 1 ). Only minor residual imbalances remained after PSM (SMD, <0.25).
Table 1 describes the patient demographics, baseline comorbidities, laboratory parameters, and medications in both groups; most variables were similar in both groups (SMD, <0.1). However, there were some residual differences: patients who underwent BS, compared with those who did not, had a higher baseline BMI (mean [SD], 43.4 [4.97] vs 42.2 [5.26]; SMD, 0.2379) and systolic blood pressure (mean [SD], 132 [16.7] vs 129 [16.0]; SMD, 0.1964), whereas the non-BS group had a higher hemoglobin A 1c than the BS group (mean [SD], 6.48% [1.65%] vs 6.15% [1.22%]; SMD, 0.2253 [to convert to proportion of total hemoglobin, multiply by 0.01]). Patients in the non-BS group had higher mean values of alanine aminotransferases than those in the BS group (39.0 [37.8] U/L vs 35.4 [31.5] U/L; SMD 0.104 [to convert to microkatal per liters, multiply by 0.0167]), whereas other values for the liver chemistries were similar. Medication use was similar in both groups ( Table 1 ).
Mean (SD) follow-up was 5.1 (1.7) years for the BS group and 4.3 (1.1) years for the non-BS group. The BS group, compared with the non-BS group, had significantly lower risk of new-onset of HF (HR, 0.60; 95% CI, 0.51-0.70), composite cardiovascular events (HR, 0.53; 95% CI, 0.44-0.65), composite cerebrovascular events (HR, 0.59; 95% CI, 0.51-0.69), and composite coronary artery interventions (HR, 0.47; 95% CI, 0.35-0.63) ( Table 2 ).
The total number of patients who remained in follow-up was 3679 patients in the BS group and 3727 patients in the non-BS group at the end of 1 year, 2092 patients in the BS group and 2133 patients at the end of 3 years, 1056 patients in the BS group and 1069 patients in the non-BS group at the end of 5 years, and 769 patients in the BS group and 516 patients in the non-BS group at the end of 7 years. Rates of incidence of new-onset HF were significantly lower in the BS group than in the matched non-BS control group ( Table 2 and Figure 2 ). Similarly, patients in the BS group had a significantly lower risk of incident composite cardiovascular events, cerebrovascular events, and coronary artery interventions than matched non-BS patients ( Figure 2 ).
Similarly, the cumulative incidence of new onset of HF at 7 years was significantly lower in the BS group (HR, 0.56; 95% CI, 0.48-0.67) ( Table 2 and Figure 2 ). BS was significantly associated with a lower hazard of reaching the other primary outcome, incident cardiovascular events (HR, 0.48; 95% CI, 0.38-0.60), composite cerebrovascular events (HR, 0.55; 95% CI, 0.46-0.65), and a composite outcome of coronary artery interventions (HR, 0.42; 95% CI, 0.31-0.58) ( Table 2 ). Kaplan-Meier survival analysis showed that the cumulative probability of being event-free up to 7 years from the index event remained significantly lower in the non-BS group compared with the BS group for all studied outcomes (log-rank P < .001) (eFigure 2 in Supplement 1 ).
Mortality was significantly lower in the BS group than the non-BS group (HR, 0.56; 95% CI, 0.42-0.74). Risks of 1-, 3-, 5- and 7-year all-cause mortality were also significantly lower in the BS group than the matched non-BS controls ( Table 2 and Figure 3 ). Kaplan-Maier survival analyses also revealed worse survival in the non-BS group compared with the BS group (log-rank P < .001) (eFigure 3 in Supplement 1 ).
Results of the sensitivity analyses are provided in eTable 1 in Supplement 1 . Results of the sensitivity analysis were consistent with the results from the primary study analysis, and all statistically significant associations remained unchanged.
In the secondary analysis comparing CVD outcomes between patients with obesity without NAFLD who underwent BS and patients with obesity without NAFLD who did not undergo BS, BS was associated with a reduction in CVD outcomes, including risk of new-onset of HF (HR, 0.40; 95% CI, 0.37-0.45), composite cardiovascular events (HR, 0.52; 95% CI, 0.46-0.60), composite cerebrovascular events (HR, 0.54; 95% CI, 0.49-0.60), and composite coronary artery interventions or surgical treatments (HR, 0.44; 95% CI, 0.36-0.53). Similar findings were observed in secondary outcome mortality (HR, 0.41; 95% CI, 0.35-0.47) (eTable 2 in Supplement 1 ).
Within 30 days after bariatric surgery, 271 patients (5.8%) experienced postoperative complications. Complications included postprocedural hemorrhage (51 patients [1.1%]), gastrointestinal leak (61 patients [1.3%]), postoperative sepsis (58 patients [1.2%]), venous thromboembolism (19 patients [0.4%]), small bowel obstruction (36 patients [0.8%]), acute postprocedural respiratory failure (12 patients [0.2%]), and acute kidney injury (53 patients [1.1%]) (eTable 3 in Supplement 1 ).
This was a large population-based, retrospective cohort study analysis that used a large sample size from a nationally representative database, statistical adjustments with a priori–identified potential confounders to balance the critical variables at the baseline by PSM, and a long duration of follow-up (7 years). Results indicate that BS was associated with decreased risk of major adverse cardiovascular events; new-onset of HF; cardiovascular events, such as unstable angina, myocardial infarction, cerebrovascular events; coronary artery interventions; and mortality. These outcomes were studied at various follow-up intervals, and consistent results were obtained at 1, 3, 5, and 7 years of follow-up.
Our findings strengthen previously reported associations and add novel data to the current literature. BS has already been reported to be associated with improved long-term adverse cardiovascular outcome risk in patients with obesity and diabetes in matched observational studies. 13 , 14 However, there is a paucity of data on the associations of BS with cardiovascular events and mortality in patients with NAFLD. A 2021 cohort study by Aminian et al 15 was the first study, to our knowledge, to observe a lower risk of major adverse cardiovascular events in their patients with biopsy-proven nonalcoholic steatohepatitis (NASH) without cirrhosis. 15 However, individual analysis of the components of the major cardiovascular events was not defined because of the small number of events, and mortality was not studied as an outcome. Notably, the findings of our large, matched cohort study suggest that BS was associated with a lower risk of all components of major adverse cardiovascular events individually and overall all-cause mortality during long-term follow-up. Moreover, the study by Aminian et al 15 only included patients with fibrotic NASH, and these findings cannot be generalizable to the broader NAFLD population. 12 Thus, the association of BS with improved CVD outcomes and mortality in an overall NAFLD population noted in our study is a novel finding. Furthermore, our analysis adds new data regarding the association of a therapeutic modality with NAFLD outcomes beyond the surrogate end points of histologic improvement or regression. The improvement in mortality observed in the BS cohort was previously unreported for any NAFLD subset, to our knowledge, and supported the use of BS as a therapeutic agent to improve clinical outcomes in NAFLD.
The elevated incidences of CVDs and mortality risk in patients with NAFLD observed in this study likely derive from the liver- and NASH-specific increase in adverse cardiovascular outcomes, as well as the other comorbid diseases that these patients tend to have. Obesity is the primary etiologic contributor to NAFLD: it develops and progresses NAFLD by fat accumulation, development of insulin resistance, activation of the immune system, and alteration in the neurohormonal state. 16 BS is the most effective obesity treatment; it reduces accumulated fat and improves peripheral insulin resistance with weight loss. 17 On the other hand, BS has also been shown to histologically improve NAFLD and NASH and reduce liver-related adverse outcomes. A study by Lassaily et al 18 found histologic resolution of NASH in 84% of patients 5 years after BS. A lower risk of progression to major liver-related adverse outcomes in patients with NASH who underwent BS compared with nonsurgical controls has been recently noted. 15 Thus, BS can be theorized to mitigate the risk of adverse cardiovascular outcomes in these patients by improving comorbidities as well as NAFLD itself. BS improves both the liver histology (NAFLD/NASH process) and the overall nonliver cardiometabolic risk factor profile. 19 , 20 Thus, it is not surprising that our study noted improved adverse cardiovascular events as well as mortality in patients with NAFLD who underwent BS.
In a secondary analysis, we compared CVD outcomes between patients with obesity without NAFLD who underwent BS and patients with obesity without NAFLD who did not undergo BS. Consistent with prior scientific data, 21 , 22 BS was associated with a reduction in adverse CVD outcomes in this comparison. The HRs were lower than those for the NAFLD cohort comparisons (primary study outcomes). These data suggest potentially lower mitigation in adverse CVD outcomes after BS in patients with NAFLD compared with patients without NAFLD with obesity undergoing BS. However, direct comparison through clinical trials and prospective comparative studies is needed to draw definitive conclusions. Patients with NAFLD constitute a higher CVD–risk cohort, and it is possible that the CVD risk reduction in this cohort by BS is secondary to obesity-related CVD risk mitigation without significant reduction in the obesity-independent CVD risk posed by NAFLD.
This study has several strengths. First, we included a robust control and adjustment for baseline and potential confounders. Second, the large sample in the propensity-matched analyses resulted in narrow CIs. It allowed us to capture a significant number of major adverse cardiovascular events and mortality incidents, which lends strength to the conclusions derived. Third, we performed a sensitivity analysis, and the statistically significant associations remained unchanged. Additionally, we performed a secondary analysis comparing CVD outcomes and mortality between patients with obesity without NAFLD who underwent BS and patients with obesity without NAFLD who did not undergo BS.
Our study also has some limitations. Data derived from EHR-based databases are susceptible to errors in coding or data entry, including misclassification and incomplete documentation. However, care was taken to use standardized measures to identify outcomes and minimize documentation errors. In addition, we have used an American Association for the Study of Liver Diseases–proposed validated diagnostic algorithm to identify individuals with NAFLD. 23 Second, although we adjusted our analyses, some residual confounding is still possible. Third, our data did not include imaging modalities (eg, conventional imaging techniques or elastography) to confirm the diagnosis of NAFLD. Severe and progressive forms of NAFLD (eg, NASH) or surrogate serum markers for fibrosis (eg, NAFLD fibrosis score, Fibrosis-4 Index, and alanine aminotransferase to aspartate aminotransferase ratio) were not evaluated. In addition, the inclusion criteria were not based on histologic diagnosis and are thus susceptible to misdiagnosis. Fourth, we did not assess outcome differences between the different metabolic surgical procedures, namely RYGB or SG. Fifth, we could not determine the association between the surgical procedure and CVD by disease phenotype because of the lack of reliable noninvasive imaging modalities for NAFLD. Similarly, we did not grade the severity of comorbid conditions at baseline, which can also result in some selection bias. However, we believe these limitations represent a minor challenge to the study’s conclusions.
The findings of this cohort study suggest that BS was associated with a lower incidence of major adverse cardiovascular events and all-cause mortality in patients with NAFLD and obesity. Although our study provides novel information, randomized clinical trials and additional observational studies are needed to corroborate our findings.
Accepted for Publication: February 23, 2023.
Published: April 7, 2023. doi:10.1001/jamanetworkopen.2023.7188
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Krishnan A et al. JAMA Network Open .
Corresponding Author: Shailendra Singh, MD, Section of Gastroenterology & Hepatology, Department of Medicine, PO Box 9161, 5th Floor HSC, Room 5500, Morgantown, WV 26506 ( [email protected] ).
Author Contributions: Dr Singh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Krishnan, Hadi, Alqahtani, Woreta, Fang, Abunnaja, Thakkar, Singh.
Acquisition, analysis, or interpretation of data: Krishnan, Hadi, Fang, Szoka, Tabone, Singh.
Drafting of the manuscript: Krishnan, Hadi, Alqahtani, Tabone, Thakkar, Singh.
Critical revision of the manuscript for important intellectual content: Krishnan, Hadi, Alqahtani, Woreta, Fang, Abunnaja, Szoka, Tabone.
Statistical analysis: Krishnan, Hadi, Fang, Singh.
Administrative, technical, or material support: Alqahtani, Tabone, Thakkar, Singh.
Supervision: Woreta, Szoka, Thakkar, Singh.
Conflict of Interest Disclosures: Dr Thakkar reported serving as a consultant for Boston Scientific Consultant, Iterative Scopes, and Medtronic outside the submitted work. No other disclosures were reported.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
More results...
Practical Issues in Wound, Skin and Ostomy Management
- Clinical Articles
- Pressure Injury
Case study: Bariatric patient with serious wounds and multiple complications
By Hedy Badolato, RD, CSR, CNSC; Denise Dacey, RD, CDE; Kim Stevens, BSN, RN, CCRN; Jen Fox, BSN, RN, CCRN; Connie Johnson, MSN, RN, WCC, LLE, OMS, DAPWCA; Hatim Youssef, DO, FCCP; and Scott Sinner, MD, FACP
Despite the healthcare team’s best efforts, not all hospitalizations go smoothly. This article describes the case of an obese patient who underwent bariatric surgery. After a 62-day hospital stay, during which a multidisciplinary team collaborated to deliver the best care possible, he died. Although the outcome certainly wasn’t what we wanted, we’d like to share his story to raise awareness of the challenges of caring for bariatric patients.
Health hazards of obesity
Obesity isn’t just a cosmetic problem; it’s a health hazard. Someone who’s 40% overweight is twice as likely to die prematurely as someone of normal weight. Obesity has been linked to many serious medical conditions, including cardiovascular disease and stroke, high blood pressure, diabetes, cancer, gallbladder disease and gallstones, osteoarthritis, gout, respiratory problems (such as sleep apnea), depression, gynecologic disorders, erectile dysfunction and other sexual health issues, nonalcoholic fatty liver disease, and metabolic syndrome (a combination of high blood glucose, high blood pressure, and high triglyceride and cholesterol levels). Obesity also causes skin problems, such as poor wound healing. In obese persons, most wounds arise secondary to poor hygiene related to obesity.
Obesity in adults is determined from body mass index (BMI). (See Defining obesity in adults .)
Understanding bariatric procedures
Bariatric procedures fall into two main categories—restrictive and malabsorptive. Restrictive procedures limit the amount of food the stomach can hold, with the goal of reducing caloric intake. Malabsorptive procedures bypass part of the small intestine, decreasing the amount of calories and nutrients the body absorbs. Some procedures are both restrictive and malabsorptive.
Restrictive bariatric procedures include laparoscopic adjustable gastric band and laparoscopic sleeve gastrectomy. Bariatric procedures that are both restrictive and malabsorptive include biliopancreatic diversion with duodenal switch (BPD-DS) and laparoscopic Roux-en-Y gastric bypass (LRYGB). Surgical complications associated with LRYGB and BPD-DS include anastomotic leaks, anastomotic strictures, and intestinal obstructions (See Visualizing the Roux-en-Y procedure .)
Importance of nutrition in bariatric patients
Various GI complications may arise after bariatric surgery, including abdominal pain, nausea, vomiting, diarrhea, hernias, and ulcers. Common nutrient deficiencies associated with such surgery involve protein, calories, calcium, iron, copper, thiamine, vitamins A, B1, B6, B12, C, D, E, and K, and folic acid.
Nutrient deficiencies depend on the length of the absorptive area and percentage of weight loss. These deficiencies progress over time. However, they can be prevented with the help of a multidisciplinary team. Unless nutrition is addressed, patients with surgical complications may experience impaired wound healing, wound dehiscence, pressure ulcers, chronic wound infections, necrotizing fasciitis, decreased cardiac and respiratory functions, increased morbidity, and even death.
Nutrition before and after surgery
Nutrition plays a critical role in skin integrity, and lack of proper nutrition can lead to pressure ulcers, necrotizing fasciitis, venous stasis ulcers, wound dehiscence, and chronic wound infections.
Preoperative diet
Adhering to a strict preoperative diet helps reduce the risk of complications. For 2 weeks before bariatric surgery, patients should consume a liquid diet, which provides several benefits:
- promotes weight loss
- helps train the brain to eat less
- helps shrink the liver, which commonly is enlarged in morbidly obese persons due to excessive intake of complex carbohydrates
- promotes postoperative healing, which helps avoid complications.
Postoperative nutrition support
After bariatric surgery, barriers to adequate nutrition include lack of physician and nutritionist collaboration, poor I.V. access, hemodynamic instability, hyperglycemia, and fluid volume imbalances. Removing these barriers requires a nutritional plan that begins before surgery.
For 24 to 48 hours after surgery, the patient should receive nutritional support (preferably enteral feedings). Such support is based on factors that contribute to physiologic stress, such as mechanical ventilation, fever, and extent of surgical wounds. Patients should receive a high-protein diet: 2 to 2.5 g/kg of ideal body weight (IBW), with 11 to 14 kcals/kg actual dry weight, or 22 to 25 kcals/kg IBW. Clinicians should stay alert for common nutrient deficiencies.
Enteral nutrition support commonly is the first choice for patients with a functional gut. Parenteral nutrition support is considered in patients with severe nausea or vomiting and gastric leaks. Ongoing monitoring of response to nutrition therapy with timely adjustment of the nutrition care plan is an important part of patient management.
The case of Gary T.
Gary T. (not his real name), a 42-year-old male, weighed 671 lb (304 kg) before LRYGB surgery; his BMI was 86.1. (Sixty days after surgery, his BMI had dropped to 66.1.) His comorbidities included diabetes, hypertension, and peripheral vascular disease.
Before and after surgery, the dietitian worked with Gary to address his nutritional needs. However, within 24 hours after surgery, his vital signs became unstable and he developed rhabdomyolysis (probably from his morbid obesity in conjunction with prolonged surgery). The nurse noted a deep-tissue injury (DTI) to the sacrum, possibly from the prolonged (7-hour) surgery and inadequate padding of the operating-room (OR) table. Eschar was firm, and the nurse applied an intact foam dressing to prevent further tissue injury.
Gary subsequently suffered multiple complications, many resulting at least partly from his poor nutritional status secondary to obesity. Below, members of the care team present their perspectives.
Intensivist Youssef
One day after surgery, Gary developed rhabdomyolysis, a syndrome of muscle necrosis with release of muscle enzymes into the circulation, leading to electrolyte imbalances and acute kidney injury. His creatine phosphokinase (CPK) level rose to 50,380 mcg/L; normal range is 0 to 235 mcg/L.
At the time, we thought his extremely high CPK level resulted from the sacral DTI secondary to prolonged surgery with the patient in one position and an inadequately padded OR table, in the setting of morbid obesity. CPK rises within 2 to 12 hours after onset of muscle injury and peaks in 24 to 72 hours; half-life is 1.5 days. CPK decreases by 40% to 50% daily unless continuous muscle injury occurs. Gary’s CPK level stayed above 1,000 mcg/L for 2 weeks and didn’t normalize until 6 weeks later. This contributed to prolonged renal failure, which persisted throughout his 2-month hospital stay.
Infectious disease specialist Sinner
Gary received prophylactic cefazolin during and immediately after surgery. We expected him to have early postoperative fevers. His initial urine, blood, and respiratory cultures were negative, as was a methicillin-resistant Staphylococcus aureus nasal swab. He also received 1 to 2 days of piperacillin-tazobactam as empiric therapy due to the fevers.
One week after surgery, Gary’s white blood cell (WBC) count rose and purulent drainage appeared in his Jackson-Pratt drain. Drainage cultures showed two modestly resistant Escherichia coli strains, a few streptococcal species, an anaerobe, and one yeast strain. GI flora were presumed to be present due to an anastomotic leak.
Four weeks after surgery, Gary’s blood cultures showed carbapenem-resistant Enterobacter , limiting his treatment options. At 5 weeks postoperatively, his necrotic decubitus ulcer was debrided; cultures were mixed but included the highly resistant Enterobacter .
At 7 weeks, Pseudomonas was isolated from another decubitus debridement; this probably resulted from his previous tigecycline therapy, to which Pseudomonas is inherently resistant (given during postop week 3). Stenotrophomonas , which is inherently resistant to carbapenems, was isolated from his sputum following carbapenem treatment. In addition, non-albicans Candida was isolated in the urine, and stemmed from his previous fluconazole therapy. Vancomycin-resistant enterococci were isolated in his blood because of his previous broad-spectrum antibiotic therapy.
Gary’s case underscores two important concepts:
- Duration and breadth of antibiotic therapy predicts which resistant organisms will be found later.
- Antibiotics can’t fix surgical problems—in this case, anastomotic leaks and necrotic ulcer tissue. Extended and broad antibiotic therapy can lead to additional complications, including excess drug toxicity, multidrug resistant infections and Clostridium difficile colitis.
Nurses Stevens and Fox
On the first postop day, Gary required bilevel positive-airway pressure treatment because he developed atelectasis from complications of prolonged surgery and inability to move due to acute postoperative pain. This condition eventually progressed to acute respiratory failure, necessitating ventilation.
Mechanical ventilation continued for 2 weeks, with multiple failed weaning attempts related to new emergence of various infections, in turn leading to a cycle of systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and multi-organ dysfunction syndrome (MODS). As a result, Gary required multiple vasopressors and continuous venovenous hemofiltration to manage hemodynamic MODS complications. After one infection was treated, another one emerged, and the cycle began again.
Two weeks after surgery, a tracheostomy was done because of Gary’s inability to wean; the goal was to reduce the risk of ventilator-associated pneumonia linked to longer intubation. Maximum duration for an internal jugular dialysis catheter is 3 weeks, due to the infection risk. Gary’s catheter had to be replaced three times during his hospital stay. He also had a left subclavian central line for central venous pressure (CVP) monitoring and vasopressor therapies. Keeping a CVP line in place too long can lead to infection, so a peripherally inserted central line was placed.
Throughout his entire stay, Gary was compromised and had abnormal laboratory values. His albumin level ranged from 1.9 to 2.2 g/dL (normal range, 3.5 to 5.2 g/dL); blood glucose ranged from 79 to 424 mg/dL (normal range, 80 to 120 mg/dL); prealbumin measured 9 mg/dL (normal range, 18 to 45 mg/dL); and WBC count ranged from 15,100 to 39,400 cells/mm3 (normal range, 4,000 to 11,000 cells/mm3). Gary’s weight ranged from 671 lb (304 kg) to 514 lb (233 kg).
Although the dietitians and physicians collaborated to develop a plan for nutritional support, Gary’s obesity and comorbidities made it difficult to gain traction in healing his wounds.
Wound care was a particular challenge. It took more than 1 hour daily to reposition Gary and clean and dress his wound with intact eschar, and nearly 2 hours for open wounds. Transporters (usually three or four at a time) aided in turning and repositioning him. A self-turning bed that assists with right and left turns was used. The wound care nurse, physicians, and a dietitian were present for daily wound care. Gary’s wife was at the bedside to provide comfort.
For patients in optimal health, debridement, pressure relief, and moisture-retentive dressings can aid wound healing. But Gary wasn’t in optimal health. When his hospital stay exceeded 30 days, he had to be transferred to another facility. The extra-special handling required for the move included an additional air mattress and additional foam protection. His situation became even more complicated and he required additional care and handling.
In the new location, Gary’s sacral wound suddenly became malodorous. The eschar was boggy but remained intact with no drainage. A partial CT scan (Gary couldn’t fit into the scanner entirely) revealed gas gangrene. Bedside sharp debridement was performed immediately. The pathology report showed Gram-positive cocci and rods—morphologies of common anaerobic organisms that can cause gangrene.
Sad outcome
Over the next several weeks, Gary’s wounds worsened. Large amounts of purulent matter continued to drain from tunnels that extended upward from undermined areas, and muscle necrosis developed. The area beneath the pannus was open and draining. Multiple surgical sites in the abdomen were open and draining foul-smelling purulent drainage. Both lower extremities developed multiple areas of necrosis. (See Declining postoperative course: Photos tell the story .)
Despite medical and nursing interventions, Gary was visibly deteriorating. On day 61, everyone involved in his care, including his wife, met and decided to withhold all life-sustaining measures. He died soon after being removed from the ventilator.
As this case demonstrates, a good patient outcome may not be possible even with optimal management of nutrition, wounds, and infection by a competent, dedicated healthcare team. When the patient continues to deteriorate, as Gary did, keeping him comfortable and maintaining his dignity take the highest priority.
Hedy Badolato and Denise Dacey are dietitians. Connie Johnson is a wound care nurse. Kim Stevens and Jen Fox are staff nurses. Hatim Youssef is an intensivist. Scott Sinner is an infectious disease specialist.
Disclaimer: The views expressed in this article are those of the author and do not necessarily represent the views of, and should not be attributed to, Wound Care Advisor. All clinical recommendations are intended to assist with determining the appropriate wound therapy for the patient. Responsibility for final decisions and actions related to care of specific patients shall remain the obligation of the institution, its staff, and the patients’ attending physicians. Nothing in this information shall be deemed to constitute the providing of medical care or the diagnosis of any medical condition. Individuals should contact their healthcare providers for medical-related information.
Related posts
Leprosy cases are rising in the US – what is the ancient disease and why is it spreading now?
Flesh-eating bacteria infections are on the rise in the US
Rare ‘Flesh-Eating’ Bacterium Spreads North as Oceans Warm
Silk wound dressing helps eliminate scar tissue formation
Predicting diabetic foot ulcer healing improves with thermal imaging
Biotech startup announces patent for regenerative tissue therapy
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals
You are here
- Volume 10, Issue 2
- Patients’ experiences of life after bariatric surgery and follow-up care: a qualitative study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-0510-4290 Karen D Coulman 1 ,
- http://orcid.org/0000-0002-5911-8172 Fiona MacKichan 1 ,
- Jane M Blazeby 1 , 2 ,
- Jenny L Donovan 1 , 3 ,
- Amanda Owen-Smith 1
- 1 Population Health Sciences, Bristol Medical School , University of Bristol , Bristol , UK
- 2 Division of Surgery, Head and Neck , University Hospitals Bristol NHS Foundation Trust , Bristol , UK
- 3 NIHR CLAHRC West , University Hospitals Bristol NHS Foundation Trust , Bristol , UK
- Correspondence to Dr Karen D Coulman; Karen.Coulman{at}bristol.ac.uk
Objectives Bariatric surgery is the most clinically effective treatment for people with severe and complex obesity, however, the psychosocial outcomes are less clear. Follow-up care after bariatric surgery is known to be important, but limited guidance exists on what this should entail, particularly related to psychological and social well-being. Patients’ perspectives are valuable to inform the design of follow-up care. This study investigated patients’ experiences of life after bariatric surgery including important aspects of follow-up care, in the long term.
Design A qualitative study using semistructured individual interviews. A constant comparative approach was used to code data and identify themes and overarching concepts.
Setting Bariatric surgery units of two publicly funded hospitals in the South of England.
Participants Seventeen adults (10 women) who underwent a primary operation for obesity (mean time since surgery 3.11 years, range 4 months to 9 years), including Roux-en-Y gastric bypass, adjustable gastric band and sleeve gastrectomy, agreed to participate in the interviews.
Results Experiences of adapting to life following surgery were characterised by the concepts of ‘normality’ and ‘ambivalence’, while experiences of ‘abandonment’ and ‘isolation’ dominated participants’ experiences of follow-up care. Patients highlighted the need for more flexible, longer-term follow-up care that addresses social and psychological difficulties postsurgery and integrates peer support.
Conclusions This research highlights unmet patient need for more accessible and holistic follow-up care that addresses the long-term multidimensional impact of bariatric surgery. Future research should investigate effective and acceptable follow-up care packages for patients undergoing bariatric surgery.
- organisation of health services
- quality in health care
- qualitative research
- adult surgery
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/ .
https://doi.org/10.1136/bmjopen-2019-035013
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
Patients who had undergone all three main types of bariatric procedures across two UK centres were included in the research.
A detailed qualitative approach was used, allowing participants to relate their own experiences in terms that were relevant for them.
A rigorous approach to analysis was undertaken, including independent coding of initial transcripts by three researchers, and agreement of emergent themes throughout analysis with at least one other qualitative researcher.
It is not known whether similar themes would be found with participants in other centres.
Findings relating to follow-up care may be less generalisable to healthcare systems with different service pathways and funding structures.
Introduction
Over 650 million or 13% of adults worldwide suffer from obesity (body mass index (BMI) ≥30 kg/m²), representing a tripling of figures since 1975. 1 Obesity is associated with an increased risk of type 2 diabetes, cardiovascular disease, certain cancers and premature death. 2 3 Within this population, people with severe and complex obesity (BMI ≥40 kg/m 2 , or 35–40 kg/m 2 with another significant health problem that could be improved by weight loss) suffer the greatest health burdens and are at the highest risk of premature death. 4 5 In addition to the physical and metabolic health burdens, people with severe and complex obesity are more likely to suffer with psychological disorders such as depression, anxiety and disordered eating, and reduced health-related quality of life (HRQL). 6 7 These individuals also suffer from social stigma and discrimination related to their weight, 6 8 which is in turn associated with adverse physical and psychological outcomes. 8 9 Thus, any interventions to treat severe and complex obesity should consider the impact on these psychosocial outcomes in addition to traditional clinical and metabolic outcomes. 10 11
Bariatric surgery, combined with behavioural change and dietary management, is the most clinically effective treatment for people with severe and complex obesity, in terms of weight loss and the improvement of comorbidities such as type 2 diabetes. 5 12 13 The three main types of bariatric operations performed in the UK include the Roux-en-Y gastric bypass (RYGB, 53.9% in 2011–13), the sleeve gastrectomy (SG, 21.4%) and the adjustable gastric band (AGB, 21.4%). 14 More recent international data indicate that the SG (46.0%) and RYGB (38.2%) are the most common bariatric operations worldwide with AGB decreasing in recent years (5.0%), and the one-anastomosis gastric bypass now gaining popularity. 15 Each of these procedures works slightly differently; mechanisms include restriction in the amount of food able to be consumed, reduction in hunger, improvement in satiety, shift in food preferences, as well as altered gut hormones, bile acids and vagal signalling. 16 While there are lots of non-randomised studies in this field, there are very few well designed and conducted randomised controlled trials with long-term follow-up. This means that true comparative assessments of RYGB, SG and AGB are absent from the literature. A current UK study has recently completed recruitment (n=1351), with the primary end point at 3 years. This will be the first pragmatic large-scale study examining all three procedures. 17
Studies which have examined HRQL after each procedure are often poorly conducted with few including baseline data and comprehensive assessments of HRQL. Some show certain aspects of HRQL to improve but not others. 11 12 18 Previous qualitative research has highlighted the complex and changeable nature of the psychosocial impact of bariatric surgery, helping to shed light on some of these inconsistencies in the HRQL literature and emphasising the importance of long-term postoperative support in helping patients manage these changes. 10 19 Previous research has also reported attendance at follow-up visits to be associated with better weight loss outcomes after bariatric surgery. 20 Follow-up care is thus important to optimise clinical and psychosocial outcomes of bariatric surgery. However, bariatric surgery follow-up care has been reported to vary greatly across the UK, 21 and current UK and US bariatric surgery guidelines focus on surgical and metabolic outcomes, with limited guidance on how to support psychological, social and lifestyle changes that affect patients’ HRQL. 5 22 Nevertheless, previous work has highlighted the importance of these multifaceted aspects of HRQL to patients who have undergone bariatric surgery and recommendations are needed on how best to support patients after surgery to optimise these outcomes. 23
In seeking to evaluate and provide recommendations on bariatric surgery follow-up care, the patient’s perspective can provide valuable information. 24 Qualitative research is useful to explore patients’ perspectives as it seeks to gain the insider’s view on how people view, experience and make sense of their social world. 25–27 The primary focus of most previous qualitative research in bariatric surgery has been on patient experiences of outcomes of surgery rather than experiences of follow-up care. 10 19 Studies that have reported on aspects of care have identified patient need for longer follow-up after bariatric surgery, better access to psychological support and the ability to communicate with health professionals between routine appointments. 19 28–36 However, most of these studies were single centre 29–32 34 36 or reported findings from select groups, such as patients that had undergone one type of bariatric procedure only (eg, AGB) 29 30 32–35 or had experienced negative outcomes such as weight regain or substance abuse issues. 28 29 32 34 A recent systematic review by Parretti et al identified few studies focusing on patients’ experiences of follow-up care after bariatric surgery in the longer term, and recommended that primary studies in this area were needed. 19 The objectives of this study were to: (1) Investigate experiences of life after bariatric surgery including follow-up care in the long-term across people who had undergone all three main types of UK bariatric procedures and (2) Use these findings to provide recommendations for follow-up care.
Patients who had undergone a primary operation for obesity at two publicly funded bariatric surgery centres in the South of England were eligible to participate in the research. Patients were identified by health professionals at each hospital using databases and clinic lists and sent information about the research. Interested patients contacted the researcher directly (KDC). For initial interviews, patients were sampled purposively, aiming for maximum variation in gender, age, starting BMI, type of operation and time since operation. Emerging findings from analysis of initial interviews guided sampling for remaining interviews. 37 Sampling continued until themes were well established with few or no new insights gained from additional data collection. 26 37 This study was undertaken as part of a wider study to develop a core outcome set for bariatric surgery (see online supplementary document S1 for protocol). 23
Supplemental material
Interviews were chosen as the method of data collection for this study due to the sensitive and complex nature of living with bariatric surgery, and to allow individual participants’ experiences to be explored in detail. Interviews were semistructured to provide some consistency in topics discussed between interviews, while allowing flexibility to adapt each interview to the participant. Thirteen participants were interviewed in their homes, four in a private research room at one of the two participating hospitals, one in a private room at the University and one over the telephone at their request. Interviews lasted between 44 and 110 min.
Written informed consent was taken and interviews conducted according to an outline topic guide, which evolved iteratively as the research progressed (see online supplementary document S2 for final version). Findings reported in this paper mainly relate to the sections of the topic guide ‘Actual outcomes of surgery’ and ‘Actual experiences of follow-up care’. Relevant demographic and clinical information were also collected ( online supplementary document S3 ). All interviews were conducted and audio recorded between February 2013 and November 2014, by a female researcher (KDC) who was a PhD student and registered dietitian. KDC underwent training in qualitative research methods and was supervised by two experienced qualitative researchers (AO-S and FM). An initial telephone conversation was held with each participant to discuss the study and arrange the interview. Participants were otherwise not previously known to the researcher prior to interview. The researcher introduced herself as a PhD student to participants. She did not reveal her professional background as a registered dietitian unprompted but did not seek to hide it if participants asked. Field notes, which provided important contextual information to aid data analysis, were made as soon as possible after each interview. 38
Recorded interviews were transcribed verbatim, and transcriptions checked for accuracy by KDC. Thematic analysis was undertaken, using techniques of constant comparison to code data and identify emerging themes. 37 39 As the aim of the study was to broadly investigate patients’ experiences of surgery, including outcomes and aspects of care, this inductive approach to analysis was chosen to ensure that themes developed were strongly linked to the data. Coding was completed for all transcripts by KDC, with a sample of transcripts independently coded by two other experienced qualitative researchers (AO-S and JLD) (see online supplementary document S4 for final coding framework). Differences in interpretation were resolved through discussion. Initial codes were built into coding structures and themes were identified. Coding and data management were facilitated using NVivo 10 software. 40 Detailed descriptive accounts were written by KDC for each small batch of interviews, which described data relating to each theme and its constituent codes. It was at this stage that relationships between themes were identified, leading to the development of higher-order categories which encompassed inter-related themes. The coding and descriptive account were completed for each batch of interviews prior to recruiting additional patients so that emerging themes could be followed up to enrich subsequent interviews. Finally, large matrices were created to compare themes and categories across all participants and summary descriptive accounts were written wherein the concepts overarching all themes and categories crystallised. 39 AO-S, FM, JLD and JMB reviewed all descriptive accounts and made suggestions about further links between themes, categories and concepts.
Patient and public involvement
The idea for this research was based on the lead author’s experience of working with patients over several years in a bariatric surgery service, as well as discussion with a representative from a relevant patient charity. This patient representative reviewed and provided feedback on the research proposal submitted for funding. After the study received funding, two patients who had undergone National Health Service-funded bariatric surgery were recruited as patient research partners and reviewed and provided feedback on the interview topic guide, and all written patient information (including study recruitment documents, and the final study summary disseminated to participants).
Of 48 patients invited, 17 agreed to take part in interviews (mean time since surgery 3.11 years, range 4 months to 9 years), although two others (spouses of existing participants) were opportunistically recruited as the research was ongoing. Twelve of the 19 participants were female, and the mean age was 51.1 years. All reported their ethnicity to be ‘White British’, and 17 had already undergone surgery ( table 1 ). The analysis presented draws on interview data from the 17 participants that had undergone surgery.
- View inline
Characteristics of participants
Bariatric surgery was a life-changing journey for participants, impacting on several different areas of their lives. The overarching concepts of ‘normality’ and ‘ambivalence’ emerged from analysis of data on patients’ experiences of adapting to life after surgery ( figure 1 ). Analysis of data relating to experiences of follow-up care was conducted separately and characterised by two concepts—‘abandonment’ and ‘isolation’ ( figure 2 ). Results are presented according to overarching concept with participant quotes used to support the description of each concept.
- Download figure
- Open in new tab
- Download powerpoint
Concepts and categories illustrating the adaptation to life after bariatric surgery including an example of supporting themes for one category.
Concepts and categories illustrating the experiences of follow-up care after bariatric surgery including supporting themes.
Adapting to life after surgery: normality and ambivalence
Throughout several areas of their lives, participants were striving to be more ‘normal’ after bariatric surgery. This related to different aspects of their lives categorised as physical health, psychological health, eating patterns and hunger, body image, weight and social functioning ( figure 1 ). Participants experienced many positive changes that undeniably brought them closer to their idea of normality. However, participants also described things that did not change, for which they still felt abnormal. Some also experienced changes perceived as negative or difficult to deal with, which made them feel more abnormal and required a process of adjustment. This was acknowledged as a ‘trade-off’ or the ‘price to pay’ (P08) for the benefits gained. The complexity of the changes experienced highlighted the ambivalence of living with the results of bariatric surgery. Despite the challenges, all participants felt the surgery was a good decision: ‘I don’t regret it for a minute. Despite all the complications and issues’ (P14).
All participants reported an improvement in activity and mobility levels and/or their ability to carry out ‘normal’ activities of daily living following surgery: ‘I’m more mobile, I can tie my shoelaces, shower properly…my life has changed for the better’ (P10). Participants also reported several positive changes related to physical and psychological health including a reduction in medications required (eg, for diabetes), an improvement in physical symptoms (such as joint pain), self-confidence and psychological well-being: ‘I feel healthier mentally in my head, like I want to get out there’ (P09).
Some participants described an improved or more ‘normal’ relationship with food after surgery, whereby they had retrained their mind to focus on ‘eating more sensibly’ rather than thinking they were ‘on a diet’ (P11). Others experienced no real change to their relationship with food, feeling as though they still had to be ‘on a permanent diet’ (P19), or continued to use food as way of coping with difficult emotions which remained: ‘I still have an awkward relationship with food…still have the same demons…I probably rely on food to deal with certain emotions’ (P14).
All 17 of the participants had lost a large amount of weight since having surgery, however, eight had regained some of this weight. Participants reported feeling distressed by this as they did not want to return to the way they were: ‘That was a real horrendous thing for me to see my weight go up a bit after all I’d gone through to get it down…’ (P07). However, a couple described being reassured by health professionals that it was normal to experience some weight regain. The majority related their weight regain to a gradual increase in appetite and/or portion sizes over time (which had initially decreased after surgery), and a feeling that the surgery was not as effective as it had been: ‘I don’t seem to be getting the urge to stop quicker, like I did before’ (P18).
The majority of participants reported developing loose-hanging excess skin following their massive weight loss, which challenged their sense of normality. Although they were pleased to be a more ‘normal’ size, some felt ashamed of how abnormal their body looked without clothes on. Skin removal surgery was a costly option, so some had learnt to live with the excess skin; however, a few found the excess skin to be particularly problematic, impacting on their mental health and relationships: ‘My husband doesn’t like the excess skin…and that’s one of the reasons why I must do something about it, because…I know I look like a bag of s**t’ (P12).
Ambivalence
Although improvements to existing health problems were important benefits of the surgery, five participants reported developing new health problems postsurgery, including micronutrient deficiencies, menstrual problems, brittle bones, low blood pressure and cardiac issues: ‘…you give up one set of health implications but you get given another set in its place…’ (P07). Some participants still suffered with several food intolerances and/or frequent gastrointestinal symptoms many years after surgery, which they reported resulted in a poorly balanced diet: ‘I can’t eat bread or meat…That’s one of the small prices I have to pay…my intake of food is nowhere near balanced…’ (P08).
Difficulties were described in developing new coping strategies to replace food, which had previously been a ‘comfort blanket’: ‘…all your insides are different but your brain…no different whatsoever…that for me was the hardest thing to adjust to, because my brain was still telling my stomach I was hungry but obviously I couldn’t [eat]…’ (P03). One patient described developing an alcohol dependency postsurgery (which they had eventually overcome), and two participants mentioned the need for more psychological input to help with their adjustment following surgery: ‘There was no formal counselling…and that might be a good idea to find out why we eat so much, why are we addicted to food…’ (P04).
Ambivalence was also evident in participants’ experiences of social functioning and stigma. Participants reported receiving positive attention due to their weight loss: ‘…people tell you ‘you look brilliant’…that is the good side of it’ (P17). For some, however, this led to mixed emotions at the revelation of ‘how negative people saw you before’ (P07). Others described receiving less negative attention and feeling less socially stigmatised due to their obesity: ‘I can walk down the road now and not get such the bad looks as I used to.’ (P04). However, a number of participants had experienced a new type of social stigma at having taken the ‘easy way out’ (P02) by having surgery (eg, not achieved weight loss through the ‘normal’ means). Some were ashamed to tell others they had undergone surgery for fear of this reaction.
Social and family eating situations could also cause anxiety for some due to attracting attention for only eating very small amounts, or unpleasant and embarrassing gastrointestinal symptoms which could arise when eating. For some this had remained an issue several years following surgery causing disruption to relationships: ‘It disrupts life because I can be eating and whether it’s the wrong food, a mouthful too much…I’ve got to go out and she can hear me retching, and it puts her off her food’ (P08). Others were able to adapt or reported their social life had ‘come back’ (P10) gradually as food tolerance improved.
Experiences of follow-up care: abandonment and isolation
Participants explained that follow-up care received after surgery was mainly provided by the specialist bariatric surgery team (although what this entailed was highly variable), with little support from their general practitioners (GPs). Only a few participants described feeling well supported overall, and all of these had undergone their surgery less than 2 years previously. However, most described at least one aspect of follow-up care which they found helpful. These included: (1) the routine monitoring of certain measures (eg, weight, nutritional blood tests); (2) the availability of one key health professional (generally a specialist dietitian or nurse), who was easy to contact on an ad hoc basis; (3) the ability to contact the bariatric team using a range of contact options (eg, telephone, email); (4) good communication between team members and (5) continuity of care (eg, being able to see the same professionals at every appointment) ( table 2 ).
Participant quotes to support positive experiences of follow-up care
Overall, however, there was a sense of abandonment and isolation in participants’ accounts of follow-up care. This related to their experiences of postoperative support from the specialist team, primary care professionals and peer support groups ( figure 2 ). Participants felt that health professionals did not always appreciate the long-term implications of life after surgery, or even if they did, services were not set up to support them adequately: ‘It happened eight years ago so no one thinks you may have any hang-ups, issues, concerns about it…the implications of the changes it makes people don't really appreciate, it’s an old record, old news’ (P07).
Abandonment
Some participants felt that problems or complications they experienced following surgery were ignored or not dealt with properly, or there was a lack of clarity of who to go to if they experienced problems. P07, for example, felt her postoperative problems were dismissed by the specialist team, and that she ‘was upsetting someone’s figures by having complications’. P12 experienced a problem with one of her surgical wounds which wouldn’t heal and wasn’t sure who to go to about it. She felt ‘quite abandoned’ and dealt with it mainly on her own. Abandonment also related to the feeling they had been given inadequate information or guidance about life following surgery: ‘They give you loads of information about what to do in the first six weeks and then there’s nothing…’ (P04).
Abandonment was also evident in accounts of support only being provided when patients themselves initiated contact: ‘I feel that as long as you didn’t contact them then you will be left alone…’ (P15). Concerns were raised for others whom they perceived less likely to seek help proactively: ‘…these people aren’t coming forward to explain that they’re having problems because they don’t want to feel like a failure…’ (P09). P18 expressed disappointment that he had not been sent any appointments post-operatively and felt he had been left ‘in limbo’ to ‘get on with it’ himself. He had not asked for help and was under the impression that it would only be appropriate to contact the team if you were having complications: ‘…obviously if I was in excruciating pain from the operation I suppose, I could have gone back…’ (P18).
Most participants also reported feeling abandoned by their GPs who were not usually supportive of them having undergone bariatric surgery and did not ‘fully appreciate the struggles that you have’ (P14) in the long-term. However, a minority of participants described feeling well-supported by their GPs who recognised the long-term health benefits of bariatric surgery: ‘…with being my dad’s doctor, he sees that hopefully I won’t have the same problems…he’s done everything he can to help me…’ (P05).
Several participants did not live locally to the hospital where the specialist team were located. This presented a barrier to accessing follow-up care, which some felt could contribute to feelings of isolation: ‘From this side of the county it’s (hospital) extremely difficult to get to…I can understand an awful lot of people thinking ’if I ring [hospital] they’re going to say come over and see me and that is so difficult to get to…I won’t bother’ (P15). Equally participants described how local primary care services were unable to support them compounding their feelings of isolation: ‘Unless they’ve (GP surgery) read my notes they don't even know I’ve got one (a gastric band)’ (P04), and ‘They (GP surgery) were very much like ‘it’s a secondary care issue, it’s not primary care’ (P07).
Isolation was also apparent in participants’ experiences of bariatric surgery peer support groups. Although not part of medical care, these represented an important source of support. These groups were typically run by patient volunteers, with limited or no input from health professionals. Some participants had access to these groups in their local areas, whereas others did not. Those unable to access a group felt this contributed to their sense of isolation postsurgery: ‘…there’s meetings where you can meet other people who’ve had the [gastric] band…but there’s no local ones for me…if people said, ‘If you do eat it, it’s going to hurt but it will go, and this is the reason it’s hurting,’ then I could have dealt with it a little bit better.’ (P17). Those that had accessed these groups described variable experiences. Some found them supportive, for example, P01 who continued to attend several years postsurgery, whereas others had negative experiences and felt quite isolated from other members. P19, for example, had disengaged from her local group which she described as being very ‘cliquey’ with members using the group mainly to emphasise negative experiences or ‘how to cheat the band’. Many felt that peer support groups including ‘a chairman’ (P15) knowledgeable in the results of bariatric surgery should be part of routine clinical care to improve accessibility of peer support and ensure consistency of information discussed.
This qualitative study found that bariatric surgery impacted participants’ physical and psychological health, eating behaviours, weight and social functioning. The overarching concepts of normality and ambivalence illustrated their lived experience following bariatric surgery. Normality was evidenced through participants’ relief at feeling more normal in some ways (eg, improved ability to undertake daily activities), yet feeling less normal in other areas, including the development of excess skin and difficulties eating ‘normally’ in social situations. Although participants experienced many positive health changes, they also experienced changes which were negative or difficult to adapt to, such as an inability to rely on emotional eating as an entrenched coping mechanism, perceived bodily deformity as a result of excess skin and the destabilisation of important relationships. These complexities highlight the ambivalence of living with the outcomes of bariatric surgery. In coping with these changes, participants received varying levels of care from specialist health professionals and GPs. Although there were some positive experiences, ‘abandonment’ and ‘isolation’ characterised most follow-up care experiences. This included feeling unsupported with postsurgery problems (other than serious complications), lack of guidance with long-term lifestyle changes, lack of understanding from GPs and limited peer support. However, all participants felt that undergoing the surgery was a good decision despite the difficulties. These findings are important in helping to define future follow-up care packages to better address the complex changes experienced after bariatric surgery.
Our findings are consistent with previous qualitative research on patient experiences of living with outcomes of bariatric surgery which depicted the complexities on patients’ sense of normality and the ‘give and take’ or ambivalent nature of the changes experienced. 10 41–43 This study strengthens the evidence for the individual and nuanced nature of how bariatric surgery changes people’s relationship with food in different ways, and changes over time, indicating the need for individualised dietary and psychological support at different time points. 10 28 41 43 44 The importance placed by participants on the social impact of bariatric surgery was also noted in a recent UK study by Graham et al . 45 These issues, including difficulties with social and family eating, should be given more attention in follow-up care. Our study confirms previous qualitative findings on the importance of continuity of care, 19 the ability to access professional advice (often from the specialist dietitian) between appointments via telephone or email, 31 the lack of psychological support after surgery 19 28–30 32 33 36 46 and the need for moderation in patient support groups. 33 34 Previous studies have related patients’ views that GPs were not equipped to adequately support them postsurgery. 19 30 31 47 This was also evident in our study with most participants describing negative experiences with GPs in relation to bariatric surgery, and feeling they were unable to offer adequate support. Despite this, several participants would have preferred to access support locally due to living remotely.
Our study expanded findings on patient experiences of bariatric surgery follow-up care as being characterised by feelings of abandonment and isolation, with views that services were not set up to support long-term issues. Abandonment was also evident in a study by Jumbe and Meyrick who described a ‘postsurgical cliff’ with patients receiving intensive support prior to bariatric surgery and then feeling abandoned after surgery. 36 Similar to our study, they described how postoperative support was reliant on patient-initiated contact. Previous research with people living with obesity suggests they may delay or avoid seeking healthcare due to societal and medical stigmas. 48 49 This has also been reported by Throsby who conducted a UK-based ethnographic study within a surgical weight management clinic. 50 She described examples of patients struggling with their eating habits and weight postsurgery, and the shame they felt at doing ‘badly’ after undergoing publicly funded surgery. The author argued that this ‘moral weight’ could lead to patients not seeking help when most needed. 50 Similarly, feelings of shame and failure at not having met the perceived postoperative expectations was one reason cited by Australian patients for non-attendance in bariatric surgery aftercare. 30
The main strength of this research is that a detailed qualitative approach to data collection was used, whereby participants were given the time and flexibility to relate their own experiences in terms that were relevant for them. A rigorous approach to analysis was undertaken, including independent coding of initial transcripts by three researchers, and discussion and agreement of emergent themes throughout analysis with at least one other qualitative researcher. A limitation of this study is the lack of ethnic diversity represented within the sample. Low numbers of people from ethnic minority groups undergo bariatric surgery in the UK (1303 between 2011 and 2013, 7.7% of total procedures), making it difficult to identify eligible people for qualitative studies. 14 A strength of this research is that we were able to over-represent male participants within our sample (41% of the 17 postoperative participants compared with 24% who undergo bariatric surgery nationally), which has been a limitation of previous qualitative studies in this area. 14 28 30 32 34–36 An additional strength was the inclusion of a clinically diverse group of patients who had undergone all three main types of bariatric procedures in the UK and who were at a broad range of time points postsurgery. Participants were also recruited from two UK centres with different follow-up programmes and health professional teams. It is not known, however, whether similar themes would be found with participants in other centres. The findings relating to follow-up care may be less generalisable to healthcare systems with different service pathways and funding structures.
Taken together with previous literature, our findings highlight that current bariatric surgery follow-up care provision is not often aligned with patient need. Patients highlighted the need for a flexible and long-term approach to follow-up care from a multidisciplinary health professional team. This includes both routine and open appointments, moderated peer support groups and different methods of contact (eg, telephone, online in addition to face to face). These recommendations are also in accordance with the recently published 2019 UK psychological guidelines for bariatric surgery which recommend a flexible and individualised approach to postoperative psychological support, including routine screening at 6–9 months postsurgery to identify support needs. 51 In addition to individual dietary and psychological support, services should consider how to better support patients in developing strategies to cope with family and social difficulties post-surgery. This may include actively engaging family and close friends in preoperative preparation and/or postoperative interventions. Future research is needed to define and evaluate an effective and acceptable follow-up care package that could be consistently applied across bariatric surgery centres. This may include the optimal systems or pathways to identify and support those who need the most help but are the least likely to seek it, ways of engaging family and social support and delivering moderated peer support groups. The relative merits of delivering follow-up care in specialist or community-based health services or how it might be shared between the two should also be investigated.
Acknowledgments
The authors would like to thank the patient research partners who advised on this research for their valuable input, as well as the study participants who gave up their time to take part in the research and the health professionals at the centres who helped with study recruitment.
- World Health Organisation
- Spadano J ,
- Coakley EH , et al
- McPherson K ,
- Marsh T , et al
- Hall K , et al
- National Institute for Health and Care Excellence
- Collins J ,
- Kolotkin RL ,
- Crosby RD ,
- Williams GR
- Owen-Smith A ,
- Donovan J ,
- Coulman KD ,
- MacKichan F ,
- Blazeby JM , et al
- Abdelrahman T ,
- Owen-Smith A , et al
- Colquitt Jill L ,
- Pickett K ,
- Loveman E , et al
- O'Brien PE ,
- MacDonald L ,
- Anderson M , et al
- Welbourn R ,
- Finlay I , et al
- Hollyman M ,
- Kinsman R , et al
- Rogers CA ,
- Reeves BC ,
- Byrne J , et al
- Lindekilde N ,
- Gladstone BP ,
- Lübeck M , et al
- Parretti HM ,
- Hughes CA ,
- Spaniolas K ,
- Kasten KR ,
- Celio A , et al
- National Confidential Enquiry into Patient Outcome and Death
- Mechanick JI ,
- Jones DB , et al
- Hopkins J ,
- Brookes ST , et al
- Richards T ,
- Montori VM ,
- Godlee F , et al
- Henderson L
- Avenell S ,
- Saules KK ,
- Wiedemann AA
- Moroshko I ,
- Brennan L ,
- Warren N , et al
- Aarts M-A ,
- Sivapalan N ,
- Nikzad S-E , et al
- Stevenson S ,
- Hill AG , et al
- Sharman M ,
- Hensher M ,
- Wilkinson S , et al
- Cleator J ,
- QSR International Pty Ltd
- Warholm C ,
- Gjengedal E ,
- Ricciardi L
- Engström M ,
- Small PK , et al
- Stolzenberger KM ,
- Meaney CA ,
- Marteka P , et al
- Nelson M , et al
- Alegria Drury CA ,
- Schumaker HD ,
- Ratcliffe D ,
- Snowdon‐Carr V
Twitter @FionaMacKichan
Contributors KDC led the study design, data collection and data analysis as part of her PhD research, and drafted this manuscript. AO-S, FM and JMB were KDC’s PhD supervisors and advised on study design, data collection and analysis, and provided comments on this manuscript. JLD advised and contributed to data analysis and provided comments on this manuscript. All authors approved the final submitted manuscript.
Funding KDC was funded by a National Institute for Health Research (NIHR) Doctoral Research Fellowship for this research project. This work was also supported by the Medical Research Council (MRC) ConDuCT-II Hub (Collaboration and innovation for Difficult and Complex randomised controlled Trials In Invasive procedures—MR/K025643/1). This publication presents independent research funded by the NIHR and the MRC. JMB is an NIHR senior investigator.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Ethical approval for the study was obtained from Northwest - Preston Research Ethics Committee (Ref 12/NW/0844).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Anonymised participant data can be made available on reasonable request to the corresponding author at [email protected].
Read the full text or download the PDF:
- Case Report
- Open access
- Published: 06 January 2021
Efficacy of laparoscopic sleeve gastrectomy for patient with morbid obesity and type 1 diabetes mellitus: a case report
- Hidetaka Ichikawa 1 ,
- Hirofumi Imoto ORCID: orcid.org/0000-0003-2037-4643 1 ,
- Naoki Tanaka 1 ,
- Hiroaki Musha 1 ,
- Shojiro Sawada 2 , 4 ,
- Takeshi Naitoh 1 , 3 ,
- Takashi Kamei 1 &
- Michiaki Unno 1
Surgical Case Reports volume 7 , Article number: 7 ( 2021 ) Cite this article
1944 Accesses
3 Citations
Metrics details
Bariatric surgery is effective for the treatment of patients with morbid obesity and type 2 diabetes mellitus (T2DM), for body weight loss and glycemic control. However, in Japan, there has been no previous report of the effectiveness bariatric surgery in a case of morbid obesity associated with acute onset type 1 diabetes mellitus (T1DM), in which pancreatic β-cells were destroyed and endogenous insulin was depleted.
Case presentation
A 36-year-old woman with morbid obesity and T1DM, diagnosed when she was 6 years, was admitted for bariatric surgery. At her first consultation, she had a body weight of 106.7 kg and a body mass index of 42.2 kg/m 2 . Her HbA1c level was 9.0%, with a required daily insulin dose of 75 units. She underwent laparoscopic sleeve gastrectomy. At 1 year after surgery, her body weight had decreased to 81.0 kg and her body mass index to 32.2 kg/m 2 . In addition, her daily required dose of insulin had decreased to 24 units, with an improvement in her HbA1c level to 7.7%.
Conclusions
Although further evidence needs to be accumulated, including long-term outcomes, laparoscopic sleeve gastrectomy may provide an effective treatment for patients with morbid obesity and T1DM for body weight loss, improvement in HbA1c level, and insulin dose reduction.
Bariatric surgery for morbid obesity is widely performed around the world [ 1 ], with demonstrated effectiveness in improving type 2 diabetes mellitus (T2DM) [ 2 , 3 ]. Furthermore, the improvement effect on glycemic control after this surgery are observed prior to body weight loss, with the metabolic effects being markedly greater than can be explained by the loss of body weight alone. In recent years, "Metabolic Surgery" has been introduced as a new concept. However, it is not clear how this concept might apply differently to type 1 diabetes mellitus (T1DM) compared to T2DM.
T1DM is a disease in which pancreatic β-cells are destroyed and insulin secretion becomes impaired. Almost in the same way as T2DM, failure of glycemic control in the chronic phase of T1DM can lead to microangiopathy (retinopathy, nephropathy, neuropathy) and macroangiopathy (atherosclerosis), which can worsen the prognostic outcomes of patients. The main treatment for T1DM is insulin therapy. In recent years, the number of patients with morbid obesity and T1DM has increased. Bae et al. reported that analyzed electronic health records in the United States estimated that 47.8% of patients with T1DM are obese [ 4 ]. Several studies on the usefulness of bariatric surgery for these cases having emerged [ 5 , 6 , 7 , 8 , 9 ]. In Japan, only two studies have described the effect of bariatric surgery on slowly progressive insulin-dependent diabetes mellitus (SPIDDM), which is included in T1DM [ 10 , 11 ], and no studies on bariatric surgery for patients with severe obesity and T1DM with insulin secretion deficiency. In this case report, we describe the effectiveness of laparoscopic sleeve gastrectomy (LSG), by reducing the size of the stomach, in a patient with morbid obesity and T1DM, without endogenous insulin, achieving weight loss, a marked reduction in insulin requirement, and improved glycemic control.
A 36-year-old Japanese female was referred to our hospital with morbid obesity and T1DM. She was diagnosed with T1DM at the age of 6 years, thereafter, treatment with multiple daily insulin was started. By the age of 20 years, she had a body weight of 70 kg, increasing to > 100 kg at the age of 34 years. Her required daily dose of insulin increased as a function of her body weight. At her initial assessment, she required 45 units of insulin aspart and 30 units of insulin glargine per day. Although a temporary weight loss and reduction in daily insulin dose was achieved with an in-hospital treatment, her weight rebounded shortly after discharge and the patient experienced difficulty in controlling her body weight. The patient expressed her intention for surgical treatment for weight loss, and she was referred to our department.
At the time of admission, her height was 159 cm and her weight 106.7 kg, BMI of 42.2 kg/m 2 . Blood analyses indicated HbA1c of 9.0%, and blood C-peptide levels were undetectable (< 0.01 ng/mL), suggesting her insulin secretion capacity was completely depleted. With medication, her blood lipid levels were within normal range. On computed tomography (CT) examination, the calculated visceral fat area was 162.6 cm 2 , with a subcutaneous fat area of 527.9 cm 2 , measured at level of the umbilicus (Fig. 1 a, b). Upper gastrointestinal endoscopy revealed no abnormalities in the esophagus, stomach, or duodenum.
Computed tomography images. a Overall image before surgery, showing b a preoperative visceral fat area of 162.6 cm 2 and subcutaneous fat area of 527.9 cm 2 . c Overall image, 1-year after the surgical procedure, showing a decrease in d the visceral fat area to 44.8 cm 2 and the subcutaneous fat area to 408.8 cm 2
To prevent complications associated with rapid postoperative blood glucose improvement, she was admitted to our hospital 2 weeks before operation for strict glycemic control, dietary restrictions, and exercise therapy. As a result, preoperative HbA1c was reduced to 7.8% and body weight was reduced to 101.1 kg.
We performed a laparoscopic sleeve gastrectomy (LSG) [ 12 ], using five ports,, as shown in Fig. 2 a. The blood vessel along the wall of the greater curvature of the stomach was first dissected. We then inserted a 36 Fr (12 mm) bougie into the stomach and resected the greater curvature of the stomach, from a point, on the oral side, 4 cm from the pylorus to the His angle, using a linear stapler. The staple line was reinforced with continuous seromuscular sutures using non-absorbable stitches (Fig. 2 b, c).
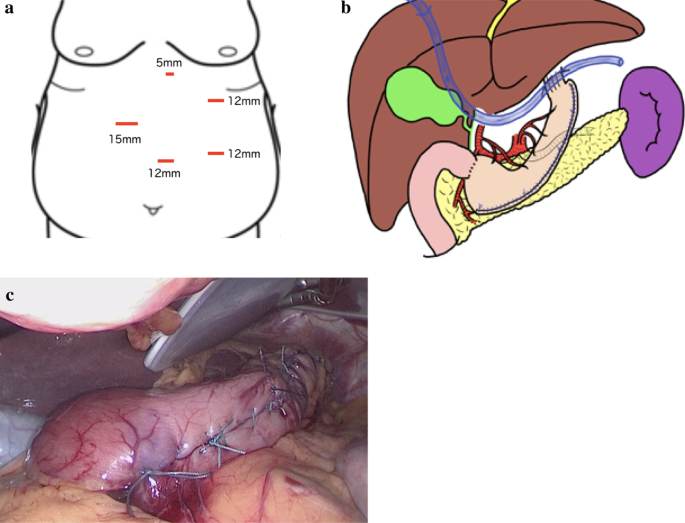
Surgical schema and gastric tube. a Schema of skin incisions (red lines), with the layout and size of ports shown. b Surgical schema, showing a drain placed below the left diaphragm. c Intraoperative photograph, with the complete gastric tube shown
After the operation, a unit of insulin aspart was mixed with 5 g of glucose contained in the infusion solution and sliding scale insulin was added as needed. From postoperative day 2, insulin glargine was administered. Sliding scale insulin was added depending on fasting blood sugar level and oral intake and her daily insulin dose was determined accordingly.
There were no postoperative complications, including severe hypoglycemic episodes. One year after the procedure, her body weight had decreased to 81.0 kg, with a BMI of 32.2 kg/m 2 , with this decrease being mainly due to a decrease in the body fat mass. Her HbA1c level improved to 7.7%, and her daily required insulin dose had been reduced to 24 units (10 units of insulin aspart and 14 units of insulin glargine per day: Fig. 3 a–d). On abdominal CT images, the visceral fat area, measured at level of the umbilicus, was 44.8 cm 2 , with a subcutaneous fat area of 408.8 cm 2 (Fig. 1 c, d). Therefore, there was a marked decrease in both visceral and subcutaneous fat.

Postoperative changes. The change, from preoperative to 12 months postoperatively, in a body weight, body mass index (BMI); b skeletal muscle mass and body fat mass; c HbA1c; and d insulin dose/day. At 1-year after the procedure, the patient’s body weight had decreased to 81 kg and her BMI to 32.2 kg/m 2 , mainly due to a decrease in body fat mass, with the skeletal muscle mass being maintained. The HbA1c level improved to 7.7%, and the daily insulin dose required reduced to 24 units
According to the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO), about 340,000 bariatric surgeries were performed, worldwide, in 2008, with this number doubling by 2016 to over 680,000, most of which were performed laparoscopically [ 1 ]. In Japan, only LSG has been covered by national insurance since 2014, with the number of LSG procedures performed having increased every year since then. It is well known that bariatric surgery is effective for weight-loss effect, as well as improving T2DM for a prolonged period after surgery [ 2 , 3 ] and lowering the risk for obesity-related diseases, such as cardiovascular disorders [ 13 ]. However, there are few reports of the therapeutic effect of LSG in patients with T1DM, and it has not yet been elucidated and remains controversial.
T1DM is caused by the destruction of pancreatic β cells due to the interaction between genetic factors, environmental factors, and autoimmune mechanisms. According to a survey of the incidence of childhood T1DM in countries around the world, the age-adjusted incidence is high in Europe and in the United States, and low in Japan, at about 2.37 per 10,000 individuals [ 14 , 15 ]. T1DM presents with a variety of clinical features and is classified into three types, according to the mode of onset: typical acute-onset type; SPIDDM, which presents with T2DM pathology at the time of diagnosis and endogenous insulin secretion gradually decreases, with progression to insulin dependence; and fulminant type, characterized by a rapid destruction of pancreatic β cells, leading to severe hyperglycemia which can sometimes be fatal. For all three types of T1DM, insulin therapy is the main treatment. Poor glycemic control over a prolonged period of time causes microangiopathy (retinopathy, nephropathy, neuropathy) and macroangiopathy (atherosclerosis), as with T2DM, with a significant negative impact on patient prognosis.
The cause of poor glycemic control in T2DM is mainly due to obesity and insulin resistance. This is important to note as the rate of obesity among adults with T1DM has been increasing. In recent years, the concept of “double diabetes” [ 16 , 17 ] has been proposed. This is a new expression of the disease in children and adolescents, with the characteristics of a mixture of the two types of diabetes as patients with T1DM diagnosed in infancy acquire the T2DM factor from adolescence to adulthood. This mixed presentation induces obesity and insulin resistance, which leads to poor glycemic control and an increase in the amount of required daily insulin.
There have been a few reports on the efficacy of bariatric surgery in patients with morbid obesity and T1DM [ 5 , 6 , 7 , 8 , 9 ]. The systematic review by Chow et al. summarizes the outcomes of bariatric surgery in 86 patients with T1DM [ 5 ]. Before surgery, the average BMI was 42.5 ± 2.65 kg/m 2 , with an average HbA1c level of 8.46 ± 0.78% and average required insulin dose of 98 ± 26 IU/day. One year after surgery, the BMI had decreased to 29.55 ± 1.76 kg/m 2 , the HbA1c level to 7.95 ± 0.55%, and the required insulin dose to 36 ± 15 IU/day. Furthermore, the risk for obesity-related diseases had also been reduced after surgery [ 8 , 9 ].
In Japan, bariatric surgery for T1DM has been reported only for cases of SPIDDM [ 10 , 11 ]; in these cases, it was possible to reduce or discontinue insulin preparations and oral glycemic drugs after surgery. As an explanatory mechanism, the authors proposed that postoperative weight loss improved insulin resistance, resulting in a protective effect on residual pancreatic β cells. However, there has been no previous report of the effectiveness bariatric surgery in a case of morbid obesity associated with typical acute-onset T1DM, in which pancreatic β-cells were destroyed and endogenous insulin was depleted. This is the first case report of typical acute-onset T1DM with endogenous insulin depletion in Japan. In this case, weight loss and improved glycemic control were achieved in the postoperative follow up period, especially the amount of daily insulin requirement was decreased more dramatically than the weight reduction. This suggests that the observed metabolic effect is not just as a result of the restrictive effect of the surgery or due to the loss in body weight alone. In considering this mechanism of improvement, the concept of “double diabetes” [ 16 , 17 ] is thought to be useful. In other words, it is presumed that the effectiveness of the bariatric surgery is mediated by an improvement in the T2DM factor among patients with double diabetes. Ashrafian et al. reported that after bariatric surgery, β cell dysfunction persisted and, thus, patient still required baseline insulin therapy, although the overall insulin requirement was reduced [ 7 ]. Incretin hormones may also play an important role. In T2DM, change in the dynamics of incretin hormone secretion, such as glucagon-like peptide-1 (GLP-1), after gastric bypass surgery, contributes to the postoperative improvement in glycemic control [ 18 ]. It is plausible that incretin hormones may also contribute to the improvement of glucose metabolism in patients with T1DM after bariatric surgery through an inhibition of glucagon secretion via α cells, even in patients without residual β cells [ 19 ]. However, the underlying mechanisms remain to be elucidated.
Our case shows the possible usefulness of bariatric surgery for the treatment of patients with morbid obesity and T1DM, without endogenous insulin, to achieve postoperative weight loss and to improve glycemic control 1 year after surgery. On the other hand, Vilarrasa et al. described that HbA1c, which had improved in the first year after surgery, returned to the preoperative baseline after 5 years [ 6 ]; therefore, our case also requires long-term strict follow-up. Accumulation of more cases and evaluation of long-term results are warranted to improve our understanding of the role of bariatric surgery for patients with obesity and T1DM.
In the short term, LSG would provide an effective treatment strategy for patients with morbid obesity and T1DM to achieve body weight loss, improve HbA1c level, and reduce the required daily insulin dose.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Abbreviations
Type 2 diabetes mellitus
- Type 1 diabetes mellitus
- Laparoscopic sleeve gastrectomy
Computed tomography
Slow progressive insulin dependent diabetes mellitus
Glucagon-like peptide-1
Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28:3783–94.
Article Google Scholar
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370:2002–133.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641–51.
Bae JP, Lage MJ, Mo D, Nelson DR, Hoogwerf BJ. Obesity and glycemic control in patients with diabetes mellitus: Analysis of physician electronic health records in the US from 2009–2011. J Diabetes Complications. 2016;30:212–20.
Article CAS Google Scholar
Chow A, Switzer NJ, Dang J, Shi X, de Gara C, Birch DW, et al. A systematic review and meta-analysis of outcomes for type 1 diabetes after bariatric surgery. J Obes. 2016;2016:6170719.
Vilarrasa N, Rubio MA, Miñambres I, Flores L, Caixàs A, Ciudin A, et al. Long-term outcomes in patients with morbid obesity and type 1 diabetes undergoing bariatric surgery. Obes Surg. 2017;27:856–63.
Ashrafian H, Harling L, Toma T, Athanasiou C, Nikiteas N, Efthimiou E, et al. Type 1 diabetes mellitus and bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2016;26:1697–704.
Brethauer SA, Aminian A, Rosenthal RJ, Kirwan JP, Kashyap SR, Schauer PR. Bariatric surgery improves the metabolic profile of morbidly obese patients with type 1 diabetes. Diabetes Care. 2014;37:E51–E5252.
Robert M, Belanger P, Hould FS, Marceau S, Tchernof A, Biertho L. Should metabolic surgery be offered in morbidly obese patients with type I diabetes? Surg Obes Relat Dis. 2015;11:798–805.
Hironaka JY, Kitahama S, Sato H, Inoue M, Takahashi T, Tamori Y. Sleeve gastrectomy induced remission of slowly progressive type 1 diabetes in a morbidly obese Japanese patient. Intern Med. 2019;58:675–8.
Uno K, Seki Y, Kasama K, Wakamatsu K, Hashimoto K, Umezawa A, et al. Mid-term results of bariatric surgery in morbidly obese Japanese patients with slow progressive autoimmune diabetes. Asian J Endosc Surg. 2018;11:238–43.
Gumbs AA, Gagner M, Dakin G, Pomp A. Sleeve gastrectomy for morbid obesity. Obes Surg. 2007;17:962–9.
Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93.
DIAMOND Project Group. Incidence and trends of childhood type 1diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–66.
Onda Y, Sugihara S, Ogata T, Yokoya S, Yokoyama T, Tajima N, et al. Incidence and prevalence of childhood-onset type 1 diabetes in Japan: the T1D study. Diabet Med. 2017;34:909–15.
Pozzilli P, Buzzetti R. A new expression of diabetes: double diabetes. Trends Endocrinol Metab. 2007;18:52–7.
Libman IM, Becker DJ. Coexistence of type 1 and type 2 diabetes mellitus: “double” diabetes? Pediatr Diabetes. 2003;4:110–3.
Laferrère B. Diabetes remission after bariatric surgery: is it just the incretins? Int J Obes Suppl. 2011;35:22–5.
Kirwan JP, Aminian A, Kashyap SR, Burguera B, Brethauer SA, Schauer PR, et al. Bariatric surgery in obese patients with type 1 diabetes. Diabetes Care. 2016;39:941–8.
Download references
Acknowledgements
We would like to thank Editage ( https://www.editage.jp ) for English language editing.
The authors declare that they have no funding.
Author information
Authors and affiliations.
Department of Surgery, Tohoku University Graduate School of Medicine, Seiryo-machi, Aoba-ku, Sendai, 980-8574, Japan
Hidetaka Ichikawa, Hirofumi Imoto, Naoki Tanaka, Hiroaki Musha, Takeshi Naitoh, Takashi Kamei & Michiaki Unno
Department of Metabolism and Diabetes, Tohoku University Graduate School of Medicine, 1-1, Seiryo-machi, Aoba-ku, Sendai, 980-8574, Japan
Shojiro Sawada
Department of Colorectal Surgery, Kitasato University School of Medicine, 1-15-1, Kitasato, Minami-ku, Sagamihara, 252-0374, Japan
Takeshi Naitoh
Department of Diabetes and Metabolism, Tohoku Medical and Pharmaceutical University, Fukumuro, Miyagino-ku, Sendai, 983-8512, Japan
You can also search for this author in PubMed Google Scholar
Contributions
HI contributed to drafting the manuscript and study design and concept. HI, HI, NT, HM, SS, TN, TK, and MU performed critical revision of the manuscript. MU provided final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Hirofumi Imoto .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ichikawa, H., Imoto, H., Tanaka, N. et al. Efficacy of laparoscopic sleeve gastrectomy for patient with morbid obesity and type 1 diabetes mellitus: a case report. surg case rep 7 , 7 (2021). https://doi.org/10.1186/s40792-020-00989-5
Download citation
Received : 11 June 2020
Accepted : 18 September 2020
Published : 06 January 2021
DOI : https://doi.org/10.1186/s40792-020-00989-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Morbid obesity
- Metabolic surgery
Bariatric Surgery - Case Study
By, Dr Harsh Sheth , Bariatric Surgeon in Mumbai
1. OVERVIEW
Bariatric surgery can be one of the most common causes of over eating or overweight. Morbid obesity is when a person has extreme amounts of excess body fat and a body mass index or BMI greater than 35. Hence regarded as surgical emergency. Hence there is a risk of Heart disease and stroke, high blood pressure and Nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH), Sleep apnea, Type 2 diabetes. Dr. Harsh Sheth specializes in performing complex bariatric surgeries and has successfully performed a variety of bariatric and laparoscopic surgery and bariatric surgery in Mumbai , in this fashion for more than ten years.
This can result in a reaction in our body in an attempt to “contain” the spread, creating obesity. This will majorly result sleep apnea, diabetes and stroke.
Dr. Harsh Sheth
She presented to us with these symptoms and described that multiple attempts to lose weight by diet and exercise had failed over the past 10 years. Which is why we offered her a bariatric surgery (Roux-en-Y gastric bypass).
She is 6 months post bariatric surgery and is leading a much better life, having lost 15% of her total body weight with diabetes resolution and reduction in her thyroid enzyme supplementation levels.
Automated page speed optimizations for fast site performance
- Skip to main content
- Skip to secondary menu
- Skip to primary sidebar
- Skip to footer

Where Wellness & Culture Connect
6 Things You Should Know Before Considering Bariatric Surgery

Bariatric surgery is a significant medical procedure that can lead to life-changing benefits for individuals struggling with obesity. If you’re considering this type of surgery, it’s important to understand what it entails, how it can impact your health, and what to expect before and after the procedure. BlackDoctor.org spoke with Shani Belgrave, M.D., a specialist in minimally invasive and bariatric surgery at Peachtree Surgical and Bariatrics, to discuss what you should know if you are considering bariatric surgery.
Dr. Belgrave, who completed her fellowship in minimally invasive and bariatric surgery at the University of Washington and joined Peachtree Surgical and Bariatrics in 2021, is a board-certified surgeon with a deep passion for helping patients transform their health. She’s been recognized for her academic achievements and research during her surgical residency at the University of Maryland and is an active mentor for medical students.
1. Bariatric Surgery is More Than a Weight Loss Tool
Bariatric surgery is more than just a tool for weight loss; it’s a powerful intervention that can significantly improve your overall health.
“Obesity, which is an excess of body fat, is associated with many health problems, including heart disease, diabetes, high cholesterol, stroke, and certain cancers. Obesity is defined by having a body mass index (BMI) over 30. Bariatric surgery significantly impacts patients’ health because, when we achieve weight loss and lower the BMI, we see a decrease in the risks of those conditions,” Dr. Belgrave shares. “Many patients even experience the resolution of issues like diabetes. Some patients can stop taking insulin after weight loss surgery, which shows how profoundly obesity affects health. It can also alleviate joint pain and urinary incontinence. Overall, bariatric surgery can transform lives.”
You May Also Like

2. You Need to Meet Specific Criteria
Before undergoing bariatric surgery, patients typically need to meet specific criteria, especially if they are using insurance to cover the procedure. Generally, a Body Mass Index (BMI) of 35 or higher, accompanied by an obesity-related health condition like diabetes or hypertension, qualifies a patient for surgery. In some cases, a BMI of 40 or more may be sufficient on its own.
The surgery itself is usually performed using minimally invasive techniques, such as laparoscopic or robotic surgery, which involve small incisions and lead to quicker recovery times. Most patients can return to work within two weeks, although those with physically demanding jobs may need a bit longer.
3. Bariatric Surgery Is Safe
There are several misconceptions about bariatric surgery that Dr. Belgrave frequently encounters. One is the belief that bariatric surgery is merely cosmetic.
“It’s not a cosmetic procedure; it’s a metabolic surgery. These surgeries impact hormones and processes in the body that help reduce fat, improving health significantly,” Dr. Belgrave notes.

Another misconception is that bariatric surgery is an “easy way out.”
“People often believe that with enough diet and exercise, they wouldn’t need surgery. But obesity is a chronic medical condition, not just a behavioral issue. It’s often genetic or environmental, in addition to behavioral. Bariatric surgery is a powerful tool to help people achieve their health goals,” Dr. Belgrave adds.
Dr. Belgrave also encounters the myth that bariatric surgery is risky.
“In truth, weight loss surgery is very safe. The safety profiles of these surgeries are excellent—comparable to gallbladder removal, which is a common procedure,” Dr. Belgrave notes.
4. The Success of the Surgery Depends on Your Commitment
While bariatric surgery can be life-changing, it’s not a standalone solution. Success depends heavily on the patient’s commitment to making lasting lifestyle changes.
“Weight loss surgery is an effective tool, but lifestyle changes are key to maintaining that success. Foods rich in carbohydrates, like bread, rice, and sugary snacks, contribute to weight gain and inflammation. Therefore, a low-carb, high-protein diet is essential. Protein helps maintain muscle and supports various bodily functions. Green vegetables are nutrient-dense and should be prioritized. Hydration is also critical—everyone should aim for 64 ounces of water daily, and sugary drinks like soda should be avoided,” Dr. Belgrave advises.
Regular exercise is another key component of post-surgery care.
“Physical activity, including cardio and strength training, is equally important. Regular exercise helps maintain weight and muscle mass, and cardio supports heart health,” Dr. Belgrave adds.
5. Follow-Up Care Is Essential
Ongoing follow-up care is vital for monitoring your progress and ensuring long-term success after bariatric surgery. Dr. Belgrave typically sees her patients at two weeks, one month, and then every few months for the first year post-surgery. After that, annual check-ups are necessary to maintain health and monitor weight.
“Since the surgeries are done through small incisions, recovery is relatively short. Most patients can return to work after two weeks, as long as there’s no heavy lifting. These follow-ups help ensure patients are doing well and maintaining their weight loss, as well as tracking health improvements,” Dr. Belgrave shares.
6. Preparation Starts Before the Surgery
If you’re considering bariatric surgery, Dr. Belgrave advises beginning to make lifestyle changes even before the procedure.
“Start by conditioning yourself for the changes that will come after surgery. Focus on eating green leafy vegetables, lean proteins like chicken and fish, and avoid processed and fried foods. Incorporate daily exercise and walking into your routine. It’s also important to consult with a bariatric surgeon to discuss the best surgery options for your individual case. Doing some research on procedures, like the sleeve gastrectomy and gastric bypass, can also help,” Dr. Belgrave advises.
Bariatric surgery is a powerful tool in the fight against obesity, offering a pathway to improved health and well-being. However, it requires a commitment to lifelong lifestyle changes and regular medical follow-up. If you’re considering this surgery, it’s important to be well-informed and prepared for the journey ahead. Consulting with an experienced bariatric surgeon like Dr. Shani Belgrave can provide the guidance and support needed to achieve the best possible outcomes.
For more information, Dr. Belgrave’s health and wellness resources are available through her website and her YouTube show and podcast, “The Coalition Talk Show” , which covers a wide range of topics to help you live your healthiest life. You can also reach her on X and Instagram .
August 22, 2024 by Jasmine Smith
The Latest In Living with Obesity

10 Important Questions To Ask Your Doctor About Weight Loss Surgery

The Best Beginner Exercises to Start Your Weight Loss Journey

Overweight vs. Obesity… Is There a Difference?

Yvette Nicole Brown Talks Obesity: “I Didn’t Know It Was a Disease”

BMI: The Mismeasure of Weight and the Mistreatment of Obesity

4 Ways Obesity Can Impact MS Patients
Where wellness & culture connect.
BDO is the world’s largest and most comprehensive online health resource specifically targeted to African Americans. BDO understands that the uniqueness of Black culture - our heritage and our traditions - plays a role in our health. BDO gives you access to innovative new approaches to the health information you need in everyday language so you can break through the disparities, gain control and live your life to its fullest.
Connect With Us
Resource centers.
- Top Blacks in Healthcare
- Clinical Trials
- Wellness on the Yard
- Immunocompromised Care
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

CASE STUDY: BARIATRIC SURGERY BOON FOR MORBID OBESITY

Related Papers
International Journal of Advanced Research (IJAR)
IJAR Indexing
Background:Bariatric surgery proved to be the only successful treatment option leading to long-term weight loss with improvement of obesity related comorbidities. The Laparoscopic Sleeve Gastrectomy (LSG) is now one of the most popular bariatric procedure worldwide with rising prevalence over last decade, while the Mini Gastric Bypass (MGB) is now gaining some popularity as a relatively new bariatric procedure Methods:The study involved forty patients; twenty of them had Laparoscopic Sleeve Gastrectomy (LSG), and twenty of them had Laparoscopic Mini Gastric Bypass (MGB). The patients were selected according to National Institute of Health (NIH) guidelines. All procedures were performed by the same team of experienced bariatric surgeons. all the patients had a one-year period of follow up after surgery and were evaluated for weight loss, morbidity (early, and late), impact on obesity associated diseases and effect on quality of life (QoL). Results:The two groups were matched considering the demographic data. Operative time was significantly longer in MGB group (P = 0.001), with mean operative time in MGB group was 74.75, while in LSG group was 53.25 min. One patient (5%) from LSG group developed stenosis and was managed by endoscopic balloon dilatation. Mean excess weight loss % after one year was 66.99% (? 1.739%) for LSG group and 67.76% (? 1.813%) for MGB group. The QoL after one year was varied between good and very good in both groups, with 70% of LSG group lie in very good category, and 75% of MGB group lie in the very good category. Conclusion:Both studied laparoscopic techniques; LSG and MGB were safe and effective, with similar results as regards significant weight loss and improvement of obesity-associated medical comorbidities and quality of life, with acceptable morbidity.
Background: Gastric size determines the amount of dietary intake which in turn determines energy intake. Body weight loss depends on negative energy balance. Objectives:The primary purpose of the present study was to assess body weight loss for 36 months in patients who underwent gastric sleeve surgery. A secondary purpose was to examine the impact of gender and age on weight changes before and after surgery. Methods: A total of 309 patients were enrolled sequentially. Age, body weight, gender, BMI, and comorbidity were assessed before and after gastric sleeve surgery for 36 months. Results: Results showed that body weight before and after surgery was influenced by both age category and gender. It was also noted that the rate of body weight reduction was faster during the 1st 12 months compared to the 2nd 12 months. Conclusions:Based on the results of the present study, it can be concluded that prior and post gastric sleeve surgery body weight is influenced by age and gender. The rate of weight reduction after gastric sleeve surgery is faster during the first 12 months, compared to the second 12 months. Gastric sleeve affected body loss and that can be considered as effective treatment for obesity and obesity related diseases.
Miroslava S
Juan Reynaldo Palomino Martinez
Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine
Winnie Tong
Annals of the Academy of Medicine, Singapore
Asim Shabbir
The Singapore National Survey of 2004 reported the prevalence of obesity to have increased to 6.9%, thus reflecting the profound changes in our society's lifestyle and eating habits. Bariatric surgery has steadily been increasing to counter the ill effects of obesity. We audited our prospective series of 31 patients who had laparoscopic adjustable gastric banding (LABG) for morbid obesity performed by our multidisciplinary team at the National University Hospital, Singapore, between August 2004 and December 2006. The median age at presentation was 40 years old including 6 males and 25 females. Their median BMI was 42.35 kg/m(2). At a median follow-up of 26 months, the median percentage of excess weight loss (%EWL) was 41.95%. The positive impact of gastric banding on comorbidities are evident whereby 15 (94%) of the diabetics had improved glycaemic control with HbA(1)C of 7.7% preoperatively improving to 5.9% postoperatively, and also 8 (58%) now take smaller doses of oral hypog...
Obesity Surgery
Sergio Vencio
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
Aristotelis Kalyvas
Nutrition in Clinical Practice
K. Isom , Kris Mogensen
BMC Surgery
Gunnar Mellgren
Isabel Matielli
João Azevedo
Clinical Nutrition
Anna Zecchinelli
SpringerPlus
Medical Clinics of North America
Olga Tucker
Federico Marchesi , Francesco Tartamella
Burhan Mayir
Nature Procedings
Jorge Farell
David Goitein , Asnat Raziel , Nasser Sakran
Surgical Endoscopy
Vladimir Schraibman
BMC Medicine
Karl Neff , Carel W le Roux
Jorge Rojas
Surgical Endoscopy and Other Interventional Techniques
belachew abe
Endocrinology
Reinhold Erben , Kerstin Stemmer
Asnat Raziel
Pediatric reports
Matteo Vandoni
Victor Rosas
Nutrition (Burbank, Los Angeles County, Calif.)
Guillermo Watkins
Annals of Surgery
Stavros-Nikolaos Karamanakos
bhanu sharadha
International Journal of Obesity
Christopher Ochner
Anne-Sophie Schneck
Zakir Mohamed
Annals of surgery
Hiroshi Sogawa, MD, FACS , Koji Hashimoto
Nature Precedings
Keren Hod , Assaf Buch , Limor Mardy-Tilbor , Tair Ben-Porat , Asnat Raziel
World Journal of Surgery
Surgery for Obesity and Related Diseases
Maddalena Leongito
Jose E Dos Santos , Carla Nonino , Julio Sergio Marchini
Christophe Moreno
British Journal of Surgery
Conor Magee
Clinical Case Reports
Gabriele Sena
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Myosteatosis predicts bariatric surgery response: A longitudinal study in patients with morbid obesity
Affiliations.
- 1 Division of Endocrinology & Metabolism, Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 2 Department of Pathology, Keimyung University School of Medicine, Daegu, Korea.
- 3 Division of Gastrointestinal Surgery, Department of Surgery, Keimyung University School of Medicine, Daegu, Korea.
- 4 Division of Gastroenterology & Hepatology, Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 5 Department of Family Medicine, Keimyung University School of Medicine, Daegu, Korea.
- PMID: 39150979
- DOI: 10.1210/clinem/dgae567
Context: Data on the preoperative factors for bariatric surgery response in patients with morbid obesity are limited, and there are no studies on the relationship between myosteatosis and surgery response.
Object: We investigated the preoperative factors determining bariatric surgery response and the impact of preoperative muscle fat infiltration on bariatric surgery response.
Methods: This retrospective longitudinal cohort study included 125 individuals (37 men, 88 women) with morbid obesity who underwent bariatric surgery. Muscle fat infiltration (skeletal muscle fat index [SMFI]) was evaluated using computed tomography-based psoas muscle mass and density at the 4th lumbar level. A bariatric surgery response was defined as ≥50% excessive weight loss at one year postoperatively.
Results: Before bariatric surgery, the patient mean body weight and body mass index (BMI) were 107.0 kg and 39.0 kg/m2, respectively. After one year, the mean body weight was 79.6 kg. The mean excessive weight loss at one year was 75.6% and 102 (81.6%) patients were categorized as responders. There were no statistically significant differences in initial BMI, age, sex, or proportion of diabetes between responders and non-responders. Responders were more likely to have lower SMFI and triglyceride and glycated hemoglobin A1c levels than non-responders at baseline (P<0.05). Multiple logistic regression analysis showed that a lower baseline SMFI was associated with bariatric surgery response (odds ratio=0.31, 95% confidence interval=0.14-0.69, P=0.004).
Conclusions: Preoperative myosteatosis may determine the response to bariatric surgery.
Keywords: bariatric surgery; fatty liver disease; myosteatosis; obesity; response.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For commercial re-use, please contact [email protected] for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact [email protected]. See the journal About page for additional terms.
PubMed Disclaimer
Similar articles
- A dynamic association between myosteatosis and liver stiffness: Results from a prospective interventional study in obese patients. Nachit M, Lanthier N, Rodriguez J, Neyrinck AM, Cani PD, Bindels LB, Hiel S, Pachikian BD, Trefois P, Thissen JP, Delzenne NM. Nachit M, et al. JHEP Rep. 2021 Jun 15;3(4):100323. doi: 10.1016/j.jhepr.2021.100323. eCollection 2021 Aug. JHEP Rep. 2021. PMID: 34355155 Free PMC article.
- Muscle fat content is strongly associated with NASH: A longitudinal study in patients with morbid obesity. Nachit M, Kwanten WJ, Thissen JP, Op De Beeck B, Van Gaal L, Vonghia L, Verrijken A, Driessen A, Horsmans Y, Francque S, Leclercq IA. Nachit M, et al. J Hepatol. 2021 Aug;75(2):292-301. doi: 10.1016/j.jhep.2021.02.037. Epub 2021 Apr 15. J Hepatol. 2021. PMID: 33865909
- Bariatric surgery: an evidence-based analysis. Medical Advisory Secretariat. Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2005;5(1):1-148. Epub 2005 Jan 1. Ont Health Technol Assess Ser. 2005. PMID: 23074460 Free PMC article.
- Predictors of remission of diabetes mellitus in severely obese individuals undergoing bariatric surgery: do BMI or procedure choice matter? A meta-analysis. Panunzi S, De Gaetano A, Carnicelli A, Mingrone G. Panunzi S, et al. Ann Surg. 2015 Mar;261(3):459-67. doi: 10.1097/SLA.0000000000000863. Ann Surg. 2015. PMID: 25361217 Review.
- The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ. Picot J, et al. Health Technol Assess. 2009 Sep;13(41):1-190, 215-357, iii-iv. doi: 10.3310/hta13410. Health Technol Assess. 2009. PMID: 19726018 Review.
Related information
Linkout - more resources, full text sources.
- Silverchair Information Systems

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Minim Access Surg
- v.17(2); Apr-Jun 2021
Complications after bariatric surgery: A multicentric study of 11,568 patients from Indian bariatric surgery outcomes reporting group
1 Centre For Metabolic Surgery, Wockhardt Hospitals, Mumbai, Maharashtra, India
Amrit Manik Nasta
Arun prasad.
2 Department of Surgery, Manipal Hospital, New Delhi, India
Gurvinder Jammu
3 Director and Chief Surgeon, Bariatric Surgery, Jammu Hospital, Jalandhar, Punjab, India
Mathias Fobi
4 Director of Clinical Affairs and Research, Mohak Bariatrics and Robotics, Indore, Madhya Pradesh, India
5 Clinical Professor of Surgery, Sri Aurobindo Medical College and Post Graduate Institute, Indore, Madhya Pradesh, India
Mohamed Ismail
6 Bariatric Surgeon, Moulana Hospital, Perintalmanna, Kerala, India
7 Bariatric Surgeon, RIMS Hospital, Kottayam, Kerala, India
Praveen Raj
8 Bariatric Surgeon, Gem Hospital and Research Institute, Coimbatore, Tamil Nadu, India
Raj Palaniappan
9 Lead Consultant, Bariatric, Metabolic and Robotic Surgery, Institute of Bariatrics, Apollo Hospitals, Chennai, Tamil Nadu, India
Sandeep Aggarwal
10 Bariatric Surgeon, AIIMS, New Delhi, India
Vivek Bindal
11 Vice-Chairman, Institute of Minimal Access, Metabolic and Bariatric Surgery, Sir Ganga Ram Hospital, New Delhi, India
Abhishek Katakwar
12 Associate Director, Laparoscopic/Robotic Bariatric and Metabolic Surgery, AIG Hospitals, Hyderabad, Telangana, India
Amar Vennapusa
13 Chief Consultant Metabolic and Bariatric Surgeon, Dr. Amar Bariatric and Metabolic Center, Hyderabad, Telangana, India
Aparna Govil Bhasker
14 Bariatric and Laparoscopic GI Surgeon, Gleneagles Global Hospital, Parel, Mumbai, Maharashtra, India
15 Bariatric and Laparoscopic GI Surgeon, Apollo Hospital, Navi Mumbai, Maharashtra, India
Atul Peters
16 HOD and Senior Consultant, Apollo Institute of Bariatric and Metabolic Surgery, Indraprastha Apollo Hospitals, New Delhi, India
17 Department of Surgical Gastroenterology, Bariatric and Metabolic Surgery, BLK Super Specialty Hospital, New Delhi, India
Digvijay Bedi
18 Hope Obesity Center, Bhopal, Madhya Pradesh, India
Jaydeep Palep
19 Department of Bariatric and Minimal Access Surgery, Nanavati Super Speciality Hospital, Mumbai, Maharashtra, India
Lakshmi Kona
20 Senior Consultant, Gleneagles Global Hospital, Hyderabad, Telangana, India
Magan Mehrotra
21 Director, Bariatric Surgery, Apex Hospital, Moradabad, Uttar Pradesh, India
Manish Baijal
22 Director, Institute of Minimal Access, Metabolic and Bariatric Surgery, Max Hospital, New Delhi, India
Mohit Bhandari
Nandakishore dukkipati.
23 Bariatric Surgeon, Livlife Hospitals, Hyderabad, Telangana, India
Randeep Wadhawan
24 Department of Minimal Access, Bariatric and Gastrointestinal Surgery, Fortis Hospital, New Delhi, India
Sarfaraz Baig
25 Department of Minimal Access Surgery, Belle Vue Clinic, Kolkata, West Bengal, India
Satish Pattanshetti
26 Ruby Hall Clinic, MJM Hospital, Pune, Maharashtra, India
Surendra Ugale
27 Director, Bariatric and Metabolic Surgery, Kirloskar and Virinchi Hospitals, Hyderabad, Telangana, India
Background:
Complications after bariatric surgery are not uncommon occurrences that influence the choice of operations both by patients and by surgeons. Complications may be classified as intra-operative, early (<30 days post-operatively) or late (beyond 30 days). The prevalence of complications is influenced by the sample size, surgeon's experience and length and percentage of follow-up. There are no multicentric reports of post-bariatric complications from India.
Objectives:
To examine the various complications after different bariatric operations that currently performed in India.
Materials and Methods:
A scientific committee designed a questionnaire to examine the post-bariatric surgery complications during a fixed time period in India. Data requested included demographic data, co-morbidities, type of procedure, complications, investigations and management of complications. This questionnaire was sent to all centres where bariatric surgery is performed in India. Data collected were reviewed, were analysed and are presented.
Twenty-four centres responded with a report on 11,568 bariatric procedures. These included 4776 (41.3%) sleeve gastrectomy (SG), 3187 (27.5%) one anastomosis gastric bypass (OAGB), 2993 (25.9%) Roux-en-Y gastric bypass (RYGB) and 612 (5.3%) other procedures. Total reported complications were 363 (3.13%). Post-operative bleeding (0.75%) and nutritional deficiency (0.75%) were the two most common complications. Leaks ( P = 0.009) and gastro-oesophageal reflux disease ( P = 0.019) were significantly higher in SG, marginal ulcers in OAGB ( P = 0.000), intestinal obstruction in RYGB ( P = 0.001) and nutritional complications in other procedures ( P = 0.000). Overall, the percentage of complications was higher in 'other' procedures (6.05%, P = 0.000). There were 18 (0.16%) reported mortalities.
Conclusions:
The post-bariatric composite complication rate from the 24 participating centres in this study from India is at par with the published data. Aggressive post-bariatric follow-up is required to improve nutritional outcomes.
INTRODUCTION
Bariatric surgery remains the single most effective long-term treatment option for obesity and its co-morbidities. The apprehension of possible complications deters even suitable candidates from undergoing a life-saving procedure, though it is widely accepted that experienced bariatric surgeons and centres of excellence have low complication rates. Further, the reporting format of complications varies across different centres and procedures. National trend analysis of bariatric-related complication rates and associated morbidity is essential to provide appropriate scientific information to physicians and the general population.
The 2016 International Federation for Surgery in Obesity and Metabolic Disorders (IFSO) report[ 1 ] included a total of 14,021 bariatric procedures from India, of which 13,765 (98.17%) were primary. Sleeve gastrectomy (SG) ( n = 8627, 62.7%) was the most commonly performed procedure followed by one anastomosis gastric bypass (OAGB) ( n = 2834, 20.6%), and Roux-en-Y gastric bypass (RYGB) ( n = 2108, 15.3%). Despite such high volumes, the reporting of surgical outcomes and multicentric post-bariatric complication data is lacking. A recent multicentre study by Baig et al .[ 2 ] on weight regain showed a high incidence of anaemia (13.9%) and hypo-albuminaemia (5.9%) after OAGB. On the other hand, Nasta et al .[ 3 ] showed no leaks, bleeds or surgical mortality after SG or RYGB. Jammu and Sharma[ 4 ] showed a leak rate of 1.5% in SG, 0.3% in RYGB and 0% in OAGB. They reported hypo-albuminaemia of 13% after OAGB in patients with biliopancreatic limb >250 cm.
Worldwide, bariatric complications and their related morbidity and mortality have reduced over the decades.[ 5 ] Although many believe that the real incidence of post-bariatric complications is high but under-reported, it is also possible that they are low, as reported in individual series.
We aimed to study the trend of post-bariatric complications amongst various procedures and correlate them with demographics and co-morbidities as well as their management, from various bariatric centres in India.
MATERIALS AND METHODS
This retrospective study was a part of a data collection exercise conducted by our centre in collaboration with the Obesity Surgery Society of India. A record of prospectively maintained data of all bariatric procedures performed for the period of January 2015–December 2017 (3 years) was collected from the primary bariatric surgeon of each centre. The requested data included demographics, co-morbidities, type of bariatric surgery, concomitant procedures and complications. In patients with complications, further details including time of diagnosis, diagnostic modality (computed tomography [CT] scan, endoscopy, biochemistry, etc.), hospitalisation period, management (conservative, endoscopic or surgical) and outcomes were recorded. In addition, each complication was defined (e.g., bleed as haemoglobin drop >2 g%) to standardise the reporting.
Data provided for patients apart from the mentioned period (2015–2017) were excluded. Patients with missing data were included for the descriptive statistics and comparison of means but were excluded from the correlation/regression analysis. Centres failing to provide data of all operated patients for the given period were excluded from the analysis.
Statistical methods
All the continuous variables were assessed for the normality using Shapiro–Wilk test. All the categorical variables were expressed either as percentage or proportion. The comparison of all the normally distributed continuous variables was done by the independent sample t -test or Welch's test depending on variance. Comparisons of all the non-normally distributed continuous variables were done by Mann–Whitney U-test, based on the number of groups. Comparisons of categorical variables were analysed by either Chi-square test or Fisher's exact, test based on the number of observations. A P < 0.05 was considered statistically significant.
Twenty-six bariatric centres contributed to this study, but the entries of two centres were excluded due to incomplete data. The remaining 24 centres reported 11,568 procedures for the period January 2015–December 2017, of which 156 (1.35%) were revisions. Thirteen centres performed over 100 surgeries annually, six centres 50–100 surgeries annually and five centres less than 50 surgeries annually. The procedure distribution is listed in Tables Tables1 1 and and2. 2 . SG was the most common procedure (4776, 41.3%) followed by OAGB (3187, 27.5%) and RYGB (2993, 25.9%). Other procedures constituted 612 (5.3%).
Distribution of different bariatric procedures
| Procedure | Total (%) | Primary (%) | Revision (%) |
|---|---|---|---|
| SG | 4776 (41.3) | 4750 (99.4) | 26 (0.6) |
| OAGB | 3187 (27.5) | 3143 (98.6) | 44 (1.38) |
| RYGB | 2993 (25.9) | 2941 (98.3) | 52 (1.73) |
| Others | 612 (5.3) | 578 (94.44) | 34 (5.55) |
| Total | 11,568 (100) | 11,412 (98.65) | 156 (1.35) |
SG: Sleeve gastrectomy, OAGB: One anastomosis gastric bypass, RYGB: Roux-en-Y gastric bypass
Distribution of other procedures ( n =612)
| Proceduree | |
|---|---|
| Adjustable band | 5 |
| SGIT | 113 |
| S-DJB | 232 |
| SADI-S | 53 |
| Sleeve with loop bi-partition | 138 |
| Intra-gastric balloon | 52 |
| Miscellaneous (band removal, bypass reversal, diagnostic laparoscopy, gastric imbrication etc.) | 19 |
| Total | 612 |
SGIT: Sleeve gastrectomy with ileal transposition, S-DJB: Sleeve with duodenojejunal bypass, SADI-S: Single anastomosis duodenoileal bypass with sleeve
Demography and co-morbidity
Overall, the mean age was 42.12 years (±12.23). The majority of the participants (54.04%) were in the age group of 31–50 years followed by 19.61% in 51–60 years. Females comprised 57.36% of all the patients [ Table 3 ].
Gender and age distribution across different procedures
| Pre-operative patient characteristics | Group 1 - RYGB | Group 2 - SG | Group 3 - OAGB | Group 4 - Others | Total |
|---|---|---|---|---|---|
| Gender, (%) | |||||
| Male | 1214 (40.66) | 1899 (39.85) | 1473 (46.25) | 333 (55.59) | 4919 (42.64) |
| Female | 1772 (59.34) | 2866 (60.15) | 1712 (53.75) | 266 (44.41) | 6616 (57.36) |
| Age, mean±SD | 43.23±12.12 | 40.44±12.29 | 43.29±12.16 | 43.67±11.02 | 42.12±12.23 |
| Age group (years), (%) | |||||
| <18 | 18 (0.61) | 60 (1.27) | 22 (0.70) | 4 (0.67) | 104 (0.91) |
| 18-30 | 484 (16.32) | 1061 (22.47) | 491 (15.59) | 71 (11.93) | 2107 (18.43) |
| 31-40 | 749 (25.26) | 1374 (29.10) | 805 (25.56) | 173 (29.08) | 3101 (27.13) |
| 41-50 | 835 (28.16) | 1150 (24.35) | 916 (29.08) | 176 (29.58) | 3077 (26.92) |
| 51-60 | 661 (22.29) | 789 (16.71) | 657 (20.86) | 135 (22.69) | 2242 (19.61) |
| 61-70 | 204 (6.88) | 263 (5.57) | 235 (7.46) | 35 (5.88) | 737 (6.45) |
| >70+ | 14 (0.47) | 25 (0.53) | 24 (0.76) | 1 (0.17) | 64 (0.56) |
| Total | 2965 (100.00) | 4722 (100.00) | 3150 (100.00) | 595 (100.00) | 11,432 (100.00) |
RYGB: Roux en Y gastric bypass, SG: Sleeve gastrectomy, OAGB: One anastomosis gastric bypass, SD: Standard deviation
Overall, the mean body mass index (BMI) was 43.74 (±7.89) kg/m2 [ Table 4 ]. Majority of patients belonged to BMI 40–49.9 (46.57%) followed by 30–39.9 kg/m2 (32.33%). Although OAGBs were preferred in BMI >50 (24.17%) as compared to the other three procedures, the difference was not significant ( P > 0.05).
Pre-operative body mass index and co-morbidity distribution across different surgical groups
| Group 1 - RYGB | Group 2 - SG | Group 3 - OAGB | Group 4 - Others | Total | |
|---|---|---|---|---|---|
| BMI, mean±SD | 43.97±7.74 | 43.32±7.65 | 44.85±7.87 | 40.03±9.14 | 43.74±7.89 |
| Co-morbidity, (%) | |||||
| Diabetes mellitus | 1124 (37.55) | 1307 (27.37) | 785 (24.63) | 259 (42.32) | 3475 (30.00) |
| Hypertension | 1239 (41.4) | 1310 (27.43) | 1039 (32.6) | 316 (51.63) | 3904 (33.70) |
| Obstructive sleep apnoea | 1114 (37.22) | 1352 (28.31) | 1299 (40.76) | 413 (67.48) | 4178 (36.10) |
| GERD | 101 (3.37) | 89 (1.86) | 13 (0.41) | 205 (33.5) | 408 (3.53) |
RYGB: Roux-en-Y gastric bypass, SG: Sleeve gastrectomy, OAGB: One anastomosis gastric bypass, SD: Standard deviation, GERD: Gastro-oesophageal reflux disease, BMI: Body mass index
62.8% ( n = 7264) of patients suffered from at least one documented co-morbidity before surgery. Amongst them, majority had obstructive sleep apnoea (OSA) (36.10%) followed by hypertension (33.70%), type 2 diabetes (T2D, 30.00%) and others [ Table 4 ].
Complications analysis
A total of 363 (3.13%) complications were reported. Leaks and gastro-oesophageal reflux disease (GERD) were significantly higher in SG – 28 (0.59%, P = 0.009 for leaks); 13 (0.27%, P = 0.019 for GERD), marginal ulcer in OAGB – 18 (0.56%, P = 0.000), intestinal obstruction in RYGB – 11 (0.37%, P = 0.001) and nutritional complications in 'other procedures' – 15 (2.45%, P = 0.000). Overall, the incidence of complications was higher in 'other procedures' – 37 (6.05%, P = 0.000) [ Table 5 ].
Distribution of complications across different surgical groups
| Complications | Group 1 - RYGB, (%) | Group 2 - SG, (%) | Group 3 - OAGB, (%) | Group 4 - Others, (%) | Total, (%) | |
|---|---|---|---|---|---|---|
| Bleed | 18 (0.6) | 41 (0.86) | 26 (0.82) | 2 (0.33) | 87 (0.75) | 0.341 |
| Leak | 9 (0.3) | 28 (0.59) | 7 (0.22) | 6 (0.98) | 50 (0.43) | 0.009 |
| Deep vein thrombosis | 1 (0.03) | 1 (0.02) | 1 (0.03) | 0 | 3 (0.03) | 0.959 |
| Pulmonary embolism | 1 (0.03) | 3 (0.06) | 3 (0.09) | 0 | 7 (0.06) | >0.05 |
| Atelectasis | 0 | 1 (0.02) | 0 | 1 (0.16) | 2 (0.02) | >0.05 |
| Intestinal obstruction | 11 (0.37) | 4 (0.08) | 1 (0.03) | 3 (0.49) | 19 (0.16) | 0.001 |
| GERD | 0 | 13 (0.27) | 6 (0.18) | 0 | 19 (0.16) | 0.019 |
| Biliary reflux | 0 | 0 | 3 (0.09) | 0 | 3 (0.03) | >0.05 |
| Marginal ulcer | 14 (0.47) | 0 | 18 (0.56) | 0 | 32 (0.28) | 0.000 |
| Nutritional | 8 (0.27) | 31 (0.65) | 30 (0.94) | 18 (2.94) | 87 (0.75) | <0.05 |
| Band erosion | 1 (0.03) | 0 | 0 | 1 (0.16) | 2 (0.02) | >0.05 |
| Any other | 17 (0.57) | 19 (0.4) | 7 (0.22) | 9 (1.47) | 52 (0.45) | 0.001 |
| Total | 81 (2.71) | 138 (2.89) | 107 (3.36) | 37 (6.05) | 363 (3.14) | 0.000 |
RYGB: Roux-en-Y gastric bypass, SG: Sleeve gastrectomy, OAGB: One anastomosis gastric bypass, GERD: Gastro-oesophageal reflux disease
Demography and complications
All complications were equally distributed across both genders ( P > 0.05). The incidence of bleeding was significantly higher in the age group >70 years (4.69%, P < 0.05) [ Table 6 ]. The incidence of leak was significantly higher (2.45%, P < 0.05) in the 25.0–29.9 BMI group [ Table 6 ].
Association of post-operative bleeding with age groups; association of post-operative leaks with BMI groups
| Age groups (years) | <18 | 18-30 | 31-40 | 41-50 | 51-60 | 61-70 | >70+ | |
|---|---|---|---|---|---|---|---|---|
| Number of bleeds, (%) | 2 (1.92) | 8 (0.38) | 19 (0.61) | 28 (0.91) | 19 (0.85) | 6 (0.81) | 3 (4.69) | <0.05 |
| ) | ||||||||
| Leak, (%) | 4 (2.45) | 12 (0.32) | 29 (0.54) | 5 (0.22) | <0.05 | |||
BMI: Body mass index
Co-morbidities and complications
Of 363 patients with complications, 202 did not have any pre-existing co-morbidity. No significant association was seen between overall complications and T2D ( P = 0.08), hypertension ( P = 0.11), OSA ( P = 0.14) and pre-operative GERD ( P = 0.07). On sub-group analysis, pre-operative GERD was significantly associated with leaks after RYGB and SG ( P < 0.05) [ Table 7 ].
Association of leak and pre-operative gastro-oesophageal reflux disease after sleeve gastrectomy and Roux-en-Y gastric bypass
| Leak after | No GERD, (%) | GERD, (%) | |
|---|---|---|---|
| RYGB | 7 (0.24) | 2 (1.98) | <0.05 |
| SG | 26 (0.55) | 2 (2.25) | <0.05 |
GERD: Gastro-oesophageal reflux disease, RYGB: Roux-en-Y gastric bypass, SG: Sleeve gastrectomy
Multivariate analysis of complications
On performing multivariate analysis, the factors significantly associated with post-operative leakage were RYGB ( P = 0.03) and presence of pre-operative GERD ( P = 0.08), while post-operative bleeding was significantly associated with OAGB ( P = 0.02). Other factors such as age, with these complications.
Primary versus revision procedures
Overall, the complications were significantly higher in revision surgery (12.18% vs. 3%, P = 0.007). Intestinal obstructions, GERD and bile reflux and nutritional deficiencies were significantly ( P < 0.05) higher in patients who underwent revision surgery. However, no significant difference was seen in the incidence of bleed, leak, deep vein thrombosis (DVT) and marginal ulcers between primary and revision surgery.
Correlation between surgical volume and complications rate
The overall complication rate was higher at centres performing <50 surgeries annually ( P = 0.000). On individual analysis, each complication except nutritional deficiencies and DVT was significantly higher in centres performing <50 surgeries annually [ Table 8 ].
Complication rates across different surgical volume centres
| Types of complications | Centres performing <50 surgeries annually | Centres performing 50-100 surgeries annually | Centres performing >100 surgeries annually | Total | |
|---|---|---|---|---|---|
| Bleed | 7 (2.81) | 10 (0.68) | 70 (0.71) | 87 (0.75) | 0.002 |
| Leak | 7 (2.81) | 8 (0.54) | 35 (0.36) | 50 (0.43) | 0.000 |
| DVT | 0 (0) | 1 (0.07) | 2 (0.02) | 3 (0.03) | 0.557 |
| Pulmonary embolism | 0 (0) | 2 (0.14) | 5 (0.05) | 7 (0.06) | >0.05 |
| Intestinal obstruction | 4 (1.61) | 2 (0.14) | 13 (0.13) | 19 (0.16) | 0.000 |
| GERD and biliary reflux | 2 (0.8) | 7 (0.47) | 13 (0.13) | 22 (0.19) | 0.000 |
| Nutritional | 3 (1.2) | 10 (0.68) | 74 (0.75) | 87 (0.75) | 0.133 |
| Marginal ulcer | 3 (1.2) | 4 (0.27) | 25 (0.25) | 32 (0.28) | 0.000 |
| Others | 5 (2.01) | 13 (0.88) | 38 (0.39) | 56 (0.48) | 0.000 |
| Total complications | 32 (12.85) | 54 (3.65) | 277 (2.82) | 363 (3.14) | 0.000 |
| Total procedures | 248 | 1481 | 9839 | 11568 |
DVT: Deep vein thrombosis, GERD: Gastro-oesophageal reflux disease
Diagnosis and management of complications
Post-operative bleeding.
Eighty (92%) patients with post-operative bleeding were diagnosed within 48 h of surgery. Imaging (CT scan/ultrasound abdomen) was performed in 44 (50.6%) patients. Forty-eight (55.2%) were managed non-surgically, 31 (35.7%) underwent re-laparoscopy, 4 (4.6%) required laparotomy and 4 (4.6%) were managed endoscopically. No mortality was reported due to bleeding in the post-operative period.
Post-operative leaks
In patients with post-operative leak, 16 (32%) were diagnosed after 7 days. The time of diagnosis was not mentioned in six patients. Imaging (CT/oral contrast/ultrasound abdomen) was performed in 41 (82%) patients, while no imaging was reported in 9 (18%). Twenty-nine (58%) underwent re-laparoscopy, 5 (10%) underwent laparotomy, 9 (18%) underwent endoscopic management, 1 (2%) underwent image-guided pigtail drainage, 2 (4%) were managed expectantly and 2 (4%) underwent combined endoscopic with laparoscopic management. Three (6%) mortalities were reported after leaks.
Post-operative nutritional deficiencies
Post-operative nutritional deficiencies were reported in 87 patients. Of these, 29 had anaemia, 35 had hypo-proteinaemia and 41 had multi-vitamin deficiencies. A few patients had both multi-vitamin and macro-nutrient deficiencies.
Thirty (34.5%) patients were diagnosed within the first 3 months, 18 (20.7%) during the 4 months to 1-year and 31 (35.6%) after the 1-year period. Seventy-five (86.2%) patients were managed with nutritional supplementation only. 12 (13.8%) patients (6 OAGBs, 4 single anastomosis duodenoileal bypass with sleeve [SADI-S], one sleeve with duodenojejunal bypass and one RYGB) required revision of limb length or reversal for severe nutritional deficiency. Overall, six mortalities were reported after nutritional complications.
Mortality was reported in 18 (0.16%) patients [ Table 9 ]. Leak ( P = 0.01) or nutritional ( P = 0.019) complications were found to be significantly associated with mortality.
Aetiological factors for mortality in post-operative period
| Group 1 - RYGB | Group 2 - SG | Group 3 - OAGB | Group 4 - Other procedures | Total | |
|---|---|---|---|---|---|
| (%) | 4 (0.13) | 4 (0.08) | 5 (0.16) | 5 (0.82) | 18 (0.16) |
| Aetiology | Leak at jejuno-jejunostomy | Mesenteric vascular thrombosis | Nutritional | Liver failure after SADI-S | |
| Leak at jejuno-jejunostomy | Nutritional | Pulmonary embolism | Nutritional (SADI-S) | ||
| Pulmonary embolism | Mesenteric panniculitis | Pulmonary embolism | Nutritional (sleeve with loop bi-partition) | ||
| Leak (site unspecified) | Pulmonary embolism | Rhabdomyolysis | Nutritional (sleeve with loop bi-partition) | ||
| Nutritional | Nutritional (sleeve with loop bi-partition) |
RYGB: Roux-en-Y gastric bypass, SG: Sleeve gastrectomy, OAGB: One anastomosis gastric bypass, SADI-S: Single anastomosis duodenoileal bypass with sleeve
This study presents the findings and analysis of 363 (3.13%) post-bariatric complications out of 11,568 surgeries from 24 centres in India.
Consistent with most published reports,[ 1 , 6 ] SG was the most commonly performed bariatric procedure. There were more OAGBs than RYGBs performed in these centres in India. Other bariatric procedures including SADI-S, sleeve with loop bipartition and SG with ileal transposition were performed in small numbers and at a few centres. 1.5% of revisions were reported in this study, as compared to 13.6% in the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) registry report.[ 7 ]
More male patients (42.64%) are undergoing bariatric surgery in India, in contrast to other countries where only 22% of men are reported to undergo surgery.[ 8 ] Although the most common BMI group in our study was 40–50 kg/m2 (46.6%), a good proportion (32.3%) belonged to 30–40 kg/m2. This may be because BMI cut-off for Asians is lower than international standards.[ 9 ] In our study, 30% of the patients suffered from T2D, 33.7% from hypertension and 36.1% from OSA in contrast to the global IFSO registry report,[ 10 ] with 19.5% patients with T2D, 30.2% with hypertension and 18.4% with sleep apnoea.
Complications
The overall complication rate in this study is 3.13% ( n = 363), similar to 2.1%–3% reported by Melissas et al .[ 6 ] from IFSO Centres of Excellence and 3.1% by Miras et al .[ 8 ] Higher complication rate of revision surgeries is reported in this study, in line with the report by Chaar et al .[ 7 ] 'Other procedures' had higher complications rates (6.05%) as compared to common procedures (2.71%, 2.89% and 3.36% for RYGB, SG and OAGB, respectively).
In this study, centres performing <50 surgeries annually had significantly more complications than centres performing higher numbers (50–100 or >100 procedures). This is in line with the study by Varban et al .,[ 11 ] who reported lower complication rates in high-volume centres (>125/year) and higher rates in low-volume centres (<50/year).
The most common complications reported were post-operative bleeding (0.75%) and nutritional deficiencies (0.75%), followed by leaks (0.43%). Subgroup analysis showed significantly higher leaks and GERD after SG, intestinal obstruction after RYGB, marginal ulcer after OAGB and nutritional deficiencies after SADI-S and sleeve with loop bipartition (other procedures). The study by Melissas et al .[ 6 ] showed bleeding as the most common complication although it was similar after SG (1.2%) and RYGB (1%). Miras et al .[ 8 ] showed post-operative vomiting and poor oral intake as the most common complication. The nutritional complications reported in this study are high, reflecting the need for improved peri-operative care and support. The study by Melissas et al .[ 6 ] showed protein malnutrition of 0.03%–0.05% after SG and RYGB. The study by Baig et al .[ 2 ] showed a hypo-albuminaemia of 2.2%–5.9% and an anaemia of 8.2%–13.9% based on different procedures.
The mortality rate in this study was 0.16% ( n = 18), while a Swedish registry study by Tao et al .[ 12 ] showed a 1-year cumulative mortality of 0.22%. In this study, leaks and nutritional deficiency were found to be significantly associated with mortality, whereas the MBSAQIP database study by Daigle et al .[ 13 ] showed venous thromboembolism, bleeding and leaks to be the major causes of mortality.
Demography and co-morbidity with complications
Complications were equally distributed across both genders in this study. The study by Stroh et al .[ 14 ] showed a higher incidence of leaks and overall complication rates in males undergoing RYGB. The age group >70 years had significantly more post-operative bleeds in our study. The Scandinavian Obesity Surgery Registry by Gerber et al .[ 15 ] showed a higher incidence of leaks and bleeds in the age group more than 50 years, while medical complications were more in the age group more than 60 years. In our study, there was no significant association of BMI with complications, except in one BMI group. In the study by Chiappetta et al .,[ 5 ] BMI did not differ significantly between patients with ( n = 503) and without complications ( n =8934). On the other hand, Sanni et al .[ 16 ] showed an increased risk of complication with every one-point increase in BMI.
Overall co-morbidities were not significantly associated with any complication. This is contrary to the American College of Surgeons database report by Abraham et al .,[ 17 ] where T2D and hypertension were significantly associated with 30-day re-admission. In our study, sub-group analysis showed that pre-existing GERD was significantly associated with leaks after SG and RYGB, whereas the study by Masoomi et al .[ 18 ] with 225,000 RYGB patients showed that the significant risk factors for leak were age > 50 years, male gender, congestive heart failure, renal failure and chronic pulmonary disease. Similarly, a study by Alizadeh et al .[ 19 ] identified an increased risk for leak in patients with oxygen dependency (adjusted odds ratio [AOR] 1.97), hypo-albuminaemia (AOR 1.66), sleep apnoea (AOR 1.52), hypertension (AOR 1.36) and T2D (AOR 1.18). We could not find any large-scale study where GERD has been linked to an increased rate of leaks.
A CECT scan was performed in 50.6% with post-operative bleed, and 55.2% of the patients with bleeding were managed conservatively. In the study by Zafar et al .[ 20 ] on bleeding after RYGB, 25.3% of patients required re-exploration, 14.9% required endoscopic management and the rest were managed conservatively. A CT/oral contrast study was performed in 82% with leak, and a laparoscopy/laparotomy was required in 68%. According to the ASMBS position statement,[ 21 ] in the clinically stable patient with a suspected leak, CT of the abdomen and pelvis with oral and intravenous contrast may have higher sensitivity and specificity than upper gastrointestinal contrast studies, with the added utility of identifying associated intra-abdominal abscesses, hernias or other pathologic conditions after RYGB or SG. Re-exploration, open or laparoscopic, is an appropriate and acceptable treatment modality when a leak is suspected and remains the diagnostic test with the highest sensitivity and specificity after RYGB and SG.
In this study, 13.8% of patients with nutritional deficiency (overall 0.1%) required revision of the limb length or reversal of the procedure. A review by Mahawar et al .[ 22 ] reported that 0.37%–0.51% of the patients after OAGB required surgical correction for nutritional deficiency. Initial reports of SADI-S have shown a conversion rate of 2.3%–3.8% for severe malnutrition.[ 23 ]
The strength of this study is twofold. It is a large volume multicentric study showing an acceptable overall complication rate, comparable to reports from other national and worldwide registries. Second, both high- and low-volume centres have participated which is a good representation of bariatric practices and post-bariatric complications in India.
There are some weaknesses in our study. As data collection exercise was started in December 2018 for patients operated in 2015–2017, long-term outcome analysis was not possible. Being a retrospective analysis, the data points could not be ascertained in advance. In addition, variation in surgical techniques of different contributing centres, which may impact complications, could not be correlated.
CONCLUSIONS
SG remains the most commonly performed procedure in India, with an upward trend in OAGB numbers. The incidence and types of complications in this study are similar to studies from other countries. Despite an acceptable complication rate, higher number of nutritional complications and complications associated with newer procedures are reported. A bariatric regulatory mechanism including institutional review for new procedures and stronger nutritional surveillance is desirable.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Estimating the Effect of Bariatric Surgery on Cardiovascular Events Using Observational Data?
Madenci, Arin L. a,b ; Kurgansky, Katherine E. c ; Dickerman, Barbra A. a ; Gerlovin, Hanna c ; Wanis, Kerollos Nashat a,d ; Smith, Ann D. c ; Trinqart, Ludovic e,f ; Gagnon, David R. c,g ; Cho, Kelly c ; Gaziano, J. Michael c,h ; Casas, Juan P. c ; Robins, James M. a,i ; Hernán, Miguel A. a,i
From the a CAUSALab, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA
b Boston Children’s Hospital and Harvard Medical School, Boston, MA
c Veterans Affairs Boston Healthcare System, Boston, MA
d Department of Surgery, Western University, London, ON
e Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, MA
f Tufts Clinical and Translational Science Institute, Tufts University, Boston, MA
g Boston University School of Public Health, Boston, MA
h Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA
i Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA.
Editors’ note: A related article appears on page 730.
Submitted October 22, 2023; accepted June 3, 2024
This research was supported by the US Department of Veterans Affairs (VA), Office of Research and Development (ORD), Cooperative Studies Program (CSP), CSP #2032, by resources and the use of facilities at the CSP Epidemiology Center at the VA Boston Healthcare System and VA Informatics and Computing Infrastructure (VINCI) (VA HSR RES 13-457). A.L.M., B.A.D., J.M.R., and M.A.H., were supported by CSP #2032.
The authors report no conflicts of interest.
The contents of this article do not represent the views of the US Department of Veterans Affairs or the US Government. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Health and Human Services and its agencies, including the Biomedical Advanced Research and Development Authority and the Food and Drug Administration, as well as any other agency of the US Government. Assumptions made within and interpretations from the analysis do not necessarily reflect the position of any US Government entity.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article ( www.epidem.com ).
The data that support the findings of this study are available from the Veterans Affairs (VA). VA data are made freely available to researchers (behind the VA firewall) with an approved VA study protocol. More information is available at https://www.virec.research.va.gov or by contacting the VA Information Research Center (VIReC) at [email protected] .
Access to the computer code used in this research is available on the CAUSALab GitHub ( github.com /CausalInference).
Correspondence: Arin L. Madenci, Department of Epidemiology, Harvard T.H. Chan School of Public Health, 677 Huntington Avenue, Boston, MA 02115. E-mail: [email protected] .
Background:
Observational studies have reported strongly protective effects of bariatric surgery on cardiovascular disease, but with oversimplified definitions of the intervention, eligibility criteria, and follow-up, which deviate from those in a randomized trial. We describe an attempt to estimate the effect of bariatric surgery on cardiovascular disease without introducing these sources of bias, which may not be entirely possible with existing observational data.
Methods:
We propose two target trials among persons with diabetes: (1) bariatric operation (vs. no operation) among individuals who have undergone preoperative preparation (lifestyle modifications and screening) and (2) preoperative preparation and a bariatric operation (vs. neither preoperative nor operative component). We emulated both target trials using observational data of US veterans.
Results:
Comparing bariatric surgery with no surgery (target trial #1; 8,087 individuals), the 7-year cardiovascular risk was 18.0% (95% CI = 6.9, 32.7) in the surgery group and 18.9% (95% CI = 17.7, 20.1) in the no-surgery group (risk difference −0.9, 95% CI = −12.0, 14.0). Comparing preoperative components plus surgery vs. neither (target trial #2; 10,065 individuals), the 7-year cardiovascular risk was 17.4% (95% CI = 13.6, 22.0) in the surgery group and 18.8% (95% CI = 17.8, 19.9) in the no-surgery group (risk difference −1.4, 95% CI = −5.1, 3.2). Body mass index and hemoglobin A1c were reduced with bariatric interventions in both emulations.
Conclusions:
Within limitations of available observational data, our estimates do not provide evidence that bariatric surgery reduces cardiovascular disease and support equipoise for a randomized trial of bariatric surgery for cardiovascular disease prevention.
Full Text Access for Subscribers:
Individual subscribers.

Institutional Users
Not a subscriber.
You can read the full text of this article if you:
- + Favorites
- View in Gallery
Readers Of this Article Also Read
A new criterion for determining a cutoff value based on the biases of incidence ..., concerning the consistency assumption in causal inference, florence nightingale: founder of modern nursing and hospital epidemiology, a structural approach to selection bias, marginal structural models to estimate the causal effect of zidovudine on the....

IMAGES
COMMENTS
Few long-term or controlled studies of bariatric surgery have been conducted to date. We report the 12-year follow-up results of an observational, prospective study of Roux-en-Y gastric bypass that...
A.W. presented to the weight center team reluctant to consider weight-loss surgery;he is a radiologist and has seen patients who have had complications from bariatric surgery. Pertinent medical history.
Bariatric surgery is an increasingly common treatment for obesity and related comorbidities. This meta-analysis aimed to compare the outcomes of bariatric surgery and medical treatment (MT).A systematic search of articles published from January 2013 to ...
The patient was evaluated by the bariatric multidisciplinary team at Sibley Memorial Hospital and approved for surgery. The patient underwent a minimally invasive Roux-en-Y gastric bypass using the latest camera technology. After her procedure, a new technique was used to test the gastro-jejunal anastomosis for any signs of a leak.
Bariatric surgery is increasingly considered for the treatment of adolescents with severe obesity, but few prospective adolescent-specific studies examining the efficacy and safety of weight-loss s...
Bariatric surgery results in weight loss and health improvements in adults and adolescents. However, whether outcomes differ according to the age of the patient at the time of surgery is unclear. W...
Although bariatric surgery is the most effective treatment for severe and complex obesity, less is known about its psychosocial impact. This systematic review synthesizes qualitative studies investigating the patient perspective of living with the outcomes ...
Chapter 10 Bariatric surgery case study An area that has seen rapid growth in the numbers of patients travelling abroad, as well as patients in the UK undergoing surgery, is bariatric or weight loss surgery. 194 The UK, like many other countries globally, has seen a rapid increase in the numbers of overweight and obese patients, with the Department of Health estimating that one-quarter of the ...
The field of metabolic/bariatric surgery has grown rapidly in the past 25 years, with observational studies and randomized controlled trials investigating a broad range of long term outcomes. Metabolic/bariatric surgery results in durable and significant weight loss and improvements in comorbid conditions, including type 2 diabetes.
Findings In this cohort study of 152 394 patients with NAFLD and obesity (including 4693 patients who underwent bariatric surgery and 147 701 patients in the nonsurgical control group), bariatric surgery was significantly associated with lower risks of major adverse cardiovascular events and all-cause mortality. These outcomes were consistent ...
This article describes the case of an obese patient who underwent bariatric surgery. After a 62-day hospital stay, during which a multidisciplinary team collaborated to deliver the best care possible, he died.
The purpose of this Evidence Analysis Library systematic review is to provide an evidence-based summary of nutrition-related practices in bariatric surgery. The systematic review methodology of the Academy of Nutrition and Dietetics was applied. A total of 27 research studies were included, analyzed, and assessed for risk of bias by trained ...
The case study below presents the outcome of a patient with long-standing borderline intellectual functioning, the optimization for surgery, and outcomes for 4.5 years postsurgery.
A study published in The Journal of Clinical Endocrinology and Metabolism states that "although the mortality rates are low, probably due to the standardization of bariatric surgical care, the complications after bariatric surgery can be deadly and must be treated promptly by surgeons familiar with these problems.".
Objectives Bariatric surgery is the most clinically effective treatment for people with severe and complex obesity, however, the psychosocial outcomes are less clear. Follow-up care after bariatric surgery is known to be important, but limited guidance exists on what this should entail, particularly related to psychological and social well-being. Patients' perspectives are valuable to inform ...
Background Bariatric surgery is effective for the treatment of patients with morbid obesity and type 2 diabetes mellitus (T2DM), for body weight loss and glycemic control. However, in Japan, there has been no previous report of the effectiveness bariatric surgery in a case of morbid obesity associated with acute onset type 1 diabetes mellitus (T1DM), in which pancreatic β-cells were destroyed ...
Summary This chapter presents the answers to the questions raised on bariatric surgery, explained with reference to the case of Abi, who presented in the outpatient clinic following a GP referral. It describes the NICE criteria for bariatric surgery. Patients need a comprehensive assessment prior to surgery and long-term follow-up (with the dietitian) in order to avoid nutritional deficiencies ...
Bariatric Surgery - Case Study. By, Dr Harsh Sheth, Bariatric Surgeon in Mumbai. 1. OVERVIEW. Bariatric surgery can be one of the most common causes of over eating or overweight. Morbid obesity is when a person has extreme amounts of excess body fat and a body mass index or BMI greater than 35. Hence regarded as surgical emergency.
Bariatric surgery is a significant medical procedure that can lead to life-changing benefits for individuals struggling with obesity. If you're considering this type of surgery, it's important to understand what it entails, how it can impact your health, and what to expect before and after the procedure.
"Bariatric surgery is an effective treatment for people with severe and complex obesity, but is definitely not an easy option, ... Case Study: Maritza Cruz Rivera, 64.
We also summarize the bariatric surgery process at Kaiser Permanente Southern California (KPSC), and then provide a detailed case study as an example of how KPSC screens patients referred for surgery, prepares them for the surgery, and cares for them once they have undergone surgery.
Bariatric surgery case study clinical decision making case study: patient with diabetes and surgery sue cummings, ms, rd, ldn dieting history. has participated
Bariatric surgery is a recognized and accepted approach for addressing weight loss and health conditions that occur as a result of morbid or severe obesity. Bariatric surgery is a generic term for weight loss surgery and is the best and effective method for enduring weight loss combined with change in lifestyle as well as diet.
The study found that for people with starting BMIs of 45 or higher, the probability of entering healthy weight category - which they defined as a BMI between 18.5 to 24.9 - was 1 in 1,667 ...
We therefore conducted a population-based study using data from nationwide Swedish registries, including information on presurgery BMI among women who had undergone bariatric surgery.
Bariatric surgery, a surgical procedure to alter the digestive system or reduce stomach size, triggers "substantial weight loss and improves lung function," according to researchers from the ...
Context: Data on the preoperative factors for bariatric surgery response in patients with morbid obesity are limited, and there are no studies on the relationship between myosteatosis and surgery response. Object: We investigated the preoperative factors determining bariatric surgery response and the impact of preoperative muscle fat infiltration on bariatric surgery response.
Bariatric surgery, also called weight loss surgery, leads to better blood sugar control and less medication use long-term in people with type 2 diabetes than non-surgical management with ...
Bariatric surgery remains the single most effective long-term treatment option for obesity and its co-morbidities. The apprehension of possible complications deters even suitable candidates from undergoing a life-saving procedure, though it is widely accepted that experienced bariatric surgeons and centres of excellence have low complication rates.
Observational studies have reported strongly protective effects of bariatric surgery on cardiovascular disease, but with oversimplified definitions of the intervention, eligibility criteria, and follow-up, which deviate from those in a randomized trial. ... Comparing bariatric surgery with no surgery (target trial #1; 8,087 individuals), the 7 ...