

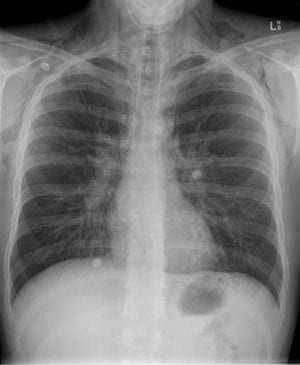
Case of Acute Severe Asthma
Kane guthrie.
- Dec 2, 2022
A 25-year-old lady Miss. Poor Compliance is rushed into your Emergency Department as a Priority 1. She is a brittle asthmatic and has been given 3x 5mg salbutamol nebs, and 0.5mg of adrenaline IM prehospital. On arrival Miss PC is sitting forward in the tripod position , using her accessory muscles to breath. She is tachypnoeic, agitated and unable to talk.
Vital signs: Pulse 143, BP 138/95, RR 42, Sp02 91% on neb, GCS 14/15.
Past Medical and Medication History
- Smoker. Severe asthmatic. Intubated twice in past 2 years
- Currently taking seritide 250/50mg, salbutamol MDI PRN and prednisolone 50mg PRN
Asthma Epidemiology
- Over 2.2 million Australians have currently diagnosed asthma
- 406 deaths attributed to asthma in 2006
- Highest risk of dying from asthma is in the elderly over 70
- The emergency clinician’s goal in treating acute severe asthma is preventing intubation
- Severe/Critical asthma is a life threatening condition
Asthma Pathophysiology
- Asthma is a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role, in particular, mast cells, eosinophils, T lymphocytes, macrophages, neutrophils, and epithelial cells.
- Smooth muscle hypertrophy and hyperplasia
- Inflammatory cell infiltration and oedema
- Goblet cell and mucous gland hyperplasia with mucous hypersecretion
- Protein deposition including collagen
- Epithelial desquamation
- Most common, responsible for 80-85% of all fatal events is characterised by eosinophilic inflammation associated with gradual deterioration over days-weeks occurring in patients with severe or poorly controlled asthma, and is slow to respond to therapy.
- The second phenotype, with neutrophilic inflammation, has both rapid onset and response to therapy.
Markers of severe asthma:
- Inability to speak in full sentences
- Use of accessory muscles or tracheal tugging
- Cyanosis and sweating
- Pulsus paradoxus (>15mmHg decreased with inspiration). With severe muscle fatigue might be absent
- Quiet chest on auscultation (The “Silent Chest”)
- Confusion or decreased level of consciousness
- Hypotension or bradycardia
- FEV 1<40% predicted
- PEF <40% of predicted or best (<25% in life threatening asthma)
- Oxygen saturation <90-92%
- PaO2 <60mmHg
- PaCO2 >45mmHg
Complications of Asthma :
- Pneumothorax, Pneumomediastinum, Pneumopericardium and Pneumoretroperitoneum
- Cardiac Arrhythmias, Myocardial ischaemia or infarction
- Electrolyte disturbances (hypokalaemia, hypomagnesaemia, hypophosphataemia)
- Lactic Acidosis
- Hyperglycaemia
Conditions that may mimic acute asthma:
- Upper airway obstruction
- Foreign-body aspiration
- Vocal cord dysfunction syndrome
- Pulmonary oedema
- Acute exacerbations of COPD
- Hysterical conversion reaction
- Munchausen syndrome
Diagnostic Test:
- Hyperinflation 5-10%
- Infiltrate 5%
- Pneumothorax <1%
- Pneumomediastinum <1%
- Respiratory alkalosis typical
- Inaccurate predictor of outcome
- Will seldom alter your treatment plan
- An objective measure of lung function
- Useful to assess response to treatment
- Impossible to obtain in the dying patient
- <25% Severe
- 25-50% Moderate
- 50-70% Mild
- >70% Discharge Goal
- Simple, and less painful than ABG
- Provides continuous oxygenation measurements
- Needs to placed on well-perfused site, difficult to obtain readings if global hypoperfusion or peripheral vasoconstriction present.
- Aim to keep sp02 >92%
Management of Acute Severe Asthma
- Hypoxia is the main cause of death in asthma
- Oxygen should be given to keep Sp02 above 92%
- A slight Pco2 rise may occur with oxygen therapy but this is of no clinical significance.
Beta-agonists:
- Rapid acting inhaled beta-agonists (bronchodilators) are the first line therapy for acute asthma.
- Nebulisers should generally be used in acute severe asthma, as provide easier delivery of medication to patient, multi dose inhalers have a role in mild to moderate asthma.
- IV salbutamol gives you the advantage of hitting the beta 2 receptors from the back door, while continuing nebulizer treatment, and should be trialed in patients not responding to nebulisers.
- Continuous nebuliser therapy appears to be more effective than intermittent nebulisers for delivering beta-agonist drugs to relieve airway spasm in acute severe asthma. (Cochrane Review, 2009)
- Salbutamol toxicity can caused a lactic acidosis which is often unrecognized in asthma patients, the lactic acidosis has been hypothesized to adversely affect ventilation by increasing ventilatory demand, increasing dead space ventilation, worsening dynamic hyperinflation and intrinsic PEEP. Management is to discontinue salbutamol at the earliest opportunity.
- Dose: Salbutamol Nebuliser Ampoule 5mg
- Dose: Salbutamol IV 5mg in 500mL of 0.9% sodium chloride or 5% dextrose start at 30mL/hr titrating up to 120mL/hr
Anticholinergics:
- Anticholinergics agents block muscarinic receptors in airway smooth muscles, inhibit vagal cholinergic tone and result in bronchodilation.
- Dose: Ipratropium bromide (Atrovent) 500ug to second dose of salbutamol via neb, can be repeated every 4hours
- Use of corticosteroids within 1 hour of presentation to an ED significantly reduces the need for hospital admission in patients with acute asthma. Benefits appear greatest in patients with more severe asthma, and those not currently receiving steroids
- Dose: Prednisolone 50mg PO
- Dose: IV Hydrocortisone 100-200mg
- Note: Parenteral route is indicated in ventilated patient or patient unable to swallow, eg. Vomiting
Adrenaline:
- Can be give either intravenously or via nebulizer
- Bronchoconstriction is the major pathology in asthma; airway oedema might also make a significant contribution. Both the a-agonist and B-agonist effects of adrenaline might be beneficial, with the alpha effect decreasing oedema and the beta effect responsible for bronchodilation.
- Dose: IV 6mg in 100mls 5% dextrose start at 1-15mLs/hour
- Dose: Nebulizer 1mg in 3ml normal saline
Aminophylline:
- The popularity of aminophylline in asthma exacerbations has diminished in recent years.
- Systematic reviews have shown that IV aminophylline in severe acute asthma does not produce additional bronchodilation above that achieved with beta-agonist and corticosteroids.
- Side effects; cardiac arrhythmia’s, vomiting, toxicity.
- Dose : 5mg/kg over 20min followed by infusion of 500mg aminophyline n 500mL of 5% dextrose at 0.5mg/kg per hour
Magnesium Sulphate:
- Magnesium potential role is asthma may involve a combination of smooth muscle relaxation, inhibition of histamine release and acetylcholine release from nerve endings.
- Most evidence to support the use of magnesium in asthma is in the acute severe asthmatic were it has been shown to be safe and beneficial.
- Dose : IV 2-4g over 30-60mins
- Heliox Mixture 80% helium/20% oxygen
- There is evidence that helium and oxygen mixtures (heliox) may provide additional benefits to patients with acute asthma.
- Heliox mixtures have the potential to decrease airway resistance, and therefore decrease the work of breathing for the severe acute asthma patient.
Antibiotics:
- Antibiotics are not indicated in the management of severe acute asthma.
- Antibiotics should only be used in the setting of an underlying pneumonia, respiratory tract infection or to aid in the prevention of ventilator-associated pneumonia in ICU.
Airway Management
Non-Invasive Positive Pressure Ventilation:
Good quality evidence and trails to support the use of NPPV in asthma are lacking, however it is worth trying when intubation is not immediately indicated. Remember the goal of the emergency clinician’s in treating asthma is to prevent intubation.
- Positive pressure is generally less than 15cmH2O
- Benefit between CPAP vs BiPAP is unknown
- Tachypnea caused by severe asthma can make it difficult for the patient to coordinate they’re breathing with machine making BiPAP uncomfortable
- Need a large randomised control trial to determine the effectives properly of NIV, in acute severe asthma.
“Asthmatic on BiPAP before being Intubated”
Mechanical Ventilation:
1-3% of acute severe asthma requires intubation. Prevention of intubation and mechanical ventilation are the goals of managing acute severe asthma, this can be achieved by maximising pre-intubation therapy, however you don’t want to wait too long or let the severe asthmatic tire before trying to intubate them. Once an asthmatic is intubated and ventilated their morbidity and mortality increasing dramatically, and it can be difficult to wean from the ventilator.
Criteria for Intubation:
- Cardiac or Respiratory arrest
- Altered mental status
- Progressive exhaustion
- Severe hypoxia despite maximal oxygen delivery
- Failure to reverse severe respiratory acidosis despite intensive therapy
- pH <7.2, carbon dioxide pressure increasing by more than 5mmHg/hr or greater than 55 to 70mm/Hg, or oxygen pressure of less than 60mm/Hg.
Challenges:
- Effective pre-oxygenation impossible
- No margin for error or delay
- Need to be intubated by most experienced person available
- High intrathoracic pressure after RSI
Recommendations:
- Fluid bolus before intubation if possible
- RSI preferred
- Ketamine for bronchodilator effects
- Permissive hypercapnea essential
Initial Ventilator settings in paralysed patients:
- FiO2 1.0, then titrate to keep SpO2 >94%
- Tidal Volume 5-6ml/kg
- Ventilator rate 6-8 breaths/min
- Long expiratory time (I:E ratio >1:2)
- Minimal PEEP < 5cmH2O
- Limit peak inspiratory pressure to <40cmH2O
- Target plateau pressure <20cmH2O
- Ensure effective humidification
- Brenner, B. Corbridge, T. & Kazzi, A. (2009). Intubation and mechanical ventilation of the asthmatic patient in respiratory failure. The Journal of Emergency Medicine. 37(2s), s23-s34.
- Camargo, C. Rachelefsky, G. & Schatz, M. (2009). Managing Asthma Exacerbation in the Emergency Department: Summary of the National Asthma Education and Prevention Program Expert Panel Report 3 Guidelines for the Management of Asthma Exacerbation.The Journal of Emergency Medicine. 37 (2S), S6-S17.
- Camargo, C. Spooner, C. & Rowe, B. (2009). Continuous versus intermittent beta-agonist for acute asthma (Review). http://www.thecochranelibrary.com.
- Chua, F. & Lai, D. (2007). Acute severe asthma: Triage, treatment and thereafter. Current Anaesthesia & Critical Care. 18, 61-68.
- Creagh-Brown, B. & Ball, J. (2007). An under-recognized complication of treatment of acute severe asthma. American Journal of Emergency Medicine. 26, 513-515.
- Hodder, R. et al. (2009). Management of acute asthma in adults in the emergency department: nonventilatory management. CMAJ. 182(2), E55-E67.
- Holley, A. & Boots, R.(2009). Review article: Management of acute severe and near-fatal asthma. Emergency Medicine Australasia, (21) 259-268.
- Jones, L. & Goodacre, S. (2009). Magnesium sulphate in the treatment of acute asthma: evaluation of current practice in adult emergency departments. Emergency Medicine Journal. 26, 783-785.
- Melnick, E. & Cottral, J. (2010). Current Guidelines for Management of Asthma in the Emergency Department. http://www.ebmedicine.net. 2(2). 1-13.
- Morris, F. & Fletcher, A. (Ed). (2009). ABC of Emergency Differential Diagnosis. Oxford: Blackwell Publishing
- National Asthma Council of Australia. Asthma management handbook: 2006. Accessed http://www.nationalasthma.org.au/cms/images/stories/amh2006_web_5.pdf, 12/02/2010
- Nowak, R. Corbridge, T. & Brenner, B. (2009). Noninvasive Ventilation. The Journal of Emergency Medicine. 37(2S), S18-S22.
- Peters, S. (2007). Continuous Bronchodilator Therapy. Chest. 131(1),1-5.
- Phipps, P. & Garrard, C. (2003). The pulmonary physician in critical care. 12: Acute severe asthma in the intensive care unit. Thorax. 58, 81-88.
- Ram, F. Wellington, S. Rowe, B. & Wedzicha, J. (2009). Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma (Review)
- Rodrigo, G. Pollack, C. Rodrigo, C. Rowe, B. (2010). Heliox for non-intubated acute asthma patents (Review).
- Rowe, B. Spooner, C. Ducharme, F. Bretzlaff, J. Bota, G. (2008). Early emergency department treatment of acute asthma with systemic corticosteroids (Review). http://www.thecochranelibrary.com.
- Rowe, B. et al. (2009). Magnesium sulfate for treating exacerbations of acute asthma in the emergency department (Review). http://www.thecochranelibrary.com.
Emergency nurse with ultra-keen interest in the realms of toxicology, sepsis, eLearning and the management of critical care in the Emergency Department | LinkedIn |
Leave a Reply Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Case report
- Open access
- Published: 21 February 2018
Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment
- Virginia Mirra 1 ,
- Silvia Montella 1 &
- Francesca Santamaria 1
BMC Pediatrics volume 18 , Article number: 73 ( 2018 ) Cite this article
11k Accesses
14 Citations
12 Altmetric
Metrics details
The primary goal of asthma management is to achieve disease control for reducing the risk of future exacerbations and progressive loss of lung function. Asthma not responding to treatment may result in significant morbidity. In many children with uncontrolled symptoms, the diagnosis of asthma may be wrong or adherence to treatment may be poor. It is then crucial to distinguish these cases from the truly “severe therapy-resistant” asthmatics by a proper filtering process. Herein we report on four cases diagnosed as difficult asthma, detail the workup that resulted in the ultimate diagnosis, and provide the process that led to the prescription of omalizumab.
Case presentation
All children had been initially referred because of asthma not responding to long-term treatment with high-dose inhaled steroids, long-acting β 2 -agonists and leukotriene receptor antagonists. Definitive diagnosis was severe asthma. Three out four patients were treated with omalizumab, which improved asthma control and patients’ quality of life. We reviewed the current literature on the diagnostic approach to the disease and on the comorbidities associated with difficult asthma and presented the perspectives on omalizumab treatment in children and adolescents. Based on the evidence from the literature review, we also proposed an algorithm for the diagnosis of pediatric difficult-to-treat and severe asthma.
Conclusions
The management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma. The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism in the management of children and adolescents with atopic severe asthma.
Peer Review reports
Children with poor asthma control have an increased risk of severe exacerbations and progressive loss of lung function, which results in the relevant use of health resources and impaired quality of life (QoL) [ 1 ]. Therefore, the primary goal of asthma management at all ages is to achieve disease control [ 2 , 3 , 4 ].
According to recent international guidelines, patients with uncontrolled asthma require a prolonged maintenance treatment with high-dose inhaled corticosteroids (ICS) in association with a long-acting β 2 -agonist (LABA) plus oral leukotriene receptor antagonist (LTRA) (Table 1 ) [ 5 ].
Nevertheless, in the presence of persistent lack of control, reversible factors such as adherence to treatment or inhalation technique should be first checked for, and diseases that can masquerade as asthma should be promptly excluded. Finally, additional strategies, in particular anti-immunoglobulin E (anti-IgE) treatment (omalizumab), are suggested for patients with moderate or severe allergic asthma that remains uncontrolled in Step 4 [ 5 ].
Herein, we reviewed the demographics, clinical presentation and treatment of four patients with uncontrolled severe asthma from our institution in order to explain why we decided to prescribe omalizumab. We also provided a review of the current literature that focuses on recent advances in the diagnosis of pediatric difficult asthma and the associated comorbidities, and summarizes the perspectives on anti-IgE treatment in children and adolescents.
Case presentations
Table 2 summarizes the clinical characteristics and the triggers/comorbidities of the cases at referral to our Institution. Unfortunately, data on psychological factors, sleep apnea, and hyperventilation syndrome were not available in any case. Clinical, lung function and airway inflammation findings at baseline and after 12 months of follow-up are reported in Table 3 . In the description of our cases, we used the terminology recommended by the ERS/ATS guidelines on severe asthma [ 6 ].
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 11, severe asthma was diagnosed. Sensitization to multiple inhalant allergens (i.e., house dust mites, dog dander, Graminaceae pollen mix, and Parietaria judaica ) and high serum IgE levels (1548 KU/l) were found. Body mass index (BMI) was within normal range. Combined treatment with increasing doses of ICS (fluticasone, up to 1000 μg/day) in association with LABA (salmeterol, 100 μg/day) plus LTRA (montelukast, 5 mg/day) has been administered over 2 years. Nevertheless, persistent symptoms and monthly hospital admissions due to asthma exacerbations despite correct inhaler technique and good adherence were reported. Parents refused to perform any test to exclude gastroesophageal reflux (GER) as comorbidity [ 6 ]. However, an ex-juvantibus 2-month-course with omeprazole was added to asthma treatment [ 7 ], but poor control persisted. Anterior rhinoscopy revealed rhinosinusitis that was treated with nasal steroids for six months [ 8 ], but asthma symptoms were unmodified. Treatment with omalizumab was added at age 12. Reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 2.0 up to 6.7 out of a maximum of 7 points) were documented over the following months. Unfortunately, after one year of treatment, adherence to omalizumab decreased because of family complaints, and eventually parents withdrew their informed consent and discontinued omalizumab. Currently, by age 17, treatment includes inhaled salmeterol/fluticasone (100 μg/500 μg∙day -1 , respectively) plus oral montelukast (10 mg/day). Satisfactory symptom control is reported, with no asthma exacerbations.
A full-term male, who had a recurrent severe preschool wheezing, at 6 years of age developed exercise-induced asthma. At age 10, severe asthma was diagnosed. High serum IgE levels (1300 KU/l) and skin prick tests positive to house dust mites were found. Despite a 3-year treatment with progressively increasing doses of inhaled fluticasone (up to 1000 μg/day) combined with salmeterol (100 μg/day) and oral montelukast (5 mg/day), monthly hospital admissions with systemic steroids use were reported. At age 13, a 24-h esophageal impedance/pH study demonstrated the presence of acid and non-acid GER [ 7 ]. Esomeprazole was added to asthma medications, but with an incomplete clinical benefit for respiratory symptoms. Esomeprazole was withdrawn after 3 months, and parents refused to re-test for GER. As respiratory symptoms persisted uncontrolled despite treatment, severe asthma was definitively diagnosed [ 6 ]. BMI was within the normal range and anterior rhinoscopy excluded rhinosinusitis. Inhaler technique and adherence were good; thus we considered the anti-IgE treatment option [ 9 ]. Subcutaneous omalizumab was started, with fast improvement of both symptoms and QoL score (from 3.9 up to 6.5). Seventeen months later, the dose of ICS had been gradually tapered and oral montelukast definitely discontinued. Currently, at age 14, treatment includes the combined administration of bimonthly subcutaneous omalizumab and of daily inhaled salmeterol/fluticasone (50 μg/100 μg∙day - 1 , respectively). Asthma control is satisfactory and no side effects are reported. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with acute respiratory failure that frequently required intensive care unit (ICU) admission. At age 6, sensitization to multiple perennial inhalant (i.e., house dust mites, dog and cat danders, Alternaria alternata , Graminaceae pollen mix, Artemisia vulgaris , Parietaria judaica , and Olea europaea pollen) and food allergens (i.e., egg, milk, and peanut) was diagnosed. Serum IgE levels were 2219 KU/l. Weight and height were appropriate for age and sex. The patient has been treated over 3 years with a combined scheme of high-dose inhaled fluticasone (up to 1000 μg/day) plus salmeterol (100 μg/day) and oral montelukast (5 mg/day), with correct inhaler technique and good adherence. Despite this, monthly hospital admissions with systemic steroids use were recorded. Rhinosinusitis and GER were excluded on the basis of appropriate testing; thus treatment with omalizumab was started when the patient was 9 years old. At age 11, adherence to treatment is satisfactory, with no side effects. More importantly, reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 6.4 to 6.8) were reported. Finally, progressive step-down of anti-asthma treatment was started, and at present (by 11.5 years) inhaled fluticasone (200 μg/day) plus bimonthly subcutaneous omalizumab provide good control of symptoms. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 4, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 8, multiple perennial inhalants and food sensitization (i.e., house dust mites, dog dander, Graminaceae pollen mix, Olea europaea pollen, tomatoes, beans, shrimps, and peas) and high serum IgE levels (1166 KU/l) were found. The patient has been treated over 5 years with inhaled fluticasone (up to 1000 μg/day) in association with salmeterol (100 μg/day) and oral montelukast (5 mg/day). Despite this, monthly hospital admissions with systemic steroids need were recorded. After checking the inhaler technique and adherence to treatment, comorbidities including obesity, rhinosinusitis and GER were excluded. Omalizumab was proposed, but parents refused it. By 13.6 years, despite a treatment including the association of inhaled salmeterol/fluticasone (100 μg/1000 μg∙day − 1 , respectively) plus oral montelukast (10 mg/day), monthly exacerbations requiring systemic steroids are reported.
Discussion and conclusions
Most children and adolescents with asthma respond well to inhaled short-acting beta 2 -agonists (SABA) on demand if symptoms are intermittent, or to low dose controller drugs plus as-needed SABA if the risk of exacerbations increases [ 1 ]. Nevertheless, a proportion of patients is referred to specialists because this strategy is not working and asthma is persistently uncontrolled [ 4 ]. For these children, assessment is primarily aimed at investigating the reasons for poor control. Indeed, when the child is initially referred, before the label of “severe, therapy-resistant asthma” (i.e., not responding to treatment even when factors as exposure to allergens and tobacco smoke have been considered) is assigned, three main categories need to be identified: 1) “not asthma at all”, in which response to treatment is suboptimal because the diagnosis is wrong; 2) “asthma plus ”, when asthma is mild but exacerbated by one or more comorbidities; and 3) “difficult-to-treat asthma”, when asthma is uncontrolled because of potentially reversible factors [ 10 ].
The reported cases highlight some aspects of the disease process that may expand the diagnosis and improve patients’ care. At our institution, the severe asthma program includes a multidisciplinary approach with consultations by gastroenterologists as well as ear, nose and throat experts. Recently, sleep medicine experts joined this multidisciplinary team; thus, unfortunately, sleep-disordered breathing (SDB) could not be excluded at the time of our patients’ assessment. Inhalation technique is periodically evaluated by nurses or doctors in each patient. Unfortunately, in Italy an individual prescription database is not available and thus we cannot assess patients’ use of medication. In two cases, the filtering process eventually identified GER and rhinosinusitis, but poor control of asthma persisted even after comorbidities were treated. In all subjects, inhaler skills, treatment adherence, and environmental exposure to indoor/outdoor allergens as well as to second- and third-hand smoke were excluded as cause of lack of control. Eventually, three out of four patients started anti-IgE treatment; asthma control was obtained and maintenance drugs were progressively reduced. In the case that refused omalizumab therapy, pulmonary function, clinical features and controller treatment including high-dose ICS were unchanged.
Previous studies have highlighted an association between increasing asthma severity in children and reduced QoL [ 11 , 12 , 13 ]. Uncontrolled asthma symptoms not only affect children physically, but can impair them socially, emotionally, and educationally [ 13 ]. In line with previous observations, 3 out 4 of our cases had poor QoL, assessed by a standardized questionnaire [ 14 ]. It is well known that improving QoL in difficult asthma is not an easy task, despite a variety of treatments aimed at achieving control [ 12 ], and much more remains to be done to address the problem. Nevertheless, 2 of our 3 cases showed a remarkable improvement of QoL after one year of treatment with omalizumab.
Reduction in forced expiratory volume in the first second (FEV 1 ) is often used to define childhood asthma severity in treatment guidelines and clinical studies [ 5 , 11 , 15 ]. Nevertheless, children with severe asthma often have a normal FEV 1 that does not improve after bronchodilators, indicating that spirometry may be a poor predictor of asthma severity in childhood [ 6 , 16 , 17 ]. Actually, children with a normal FEV 1 , both before and after β 2 -agonist, may show a bronchodilator response in terms of forced expiratory flow between 25% and 75% (FEF 25–75 ) [ 18 ]. However, the utility of FEF 25–75 in the assessment or treatment of severe asthma is currently unknown. Interestingly, all the reported cases showed normal or slightly reduced values of FEV 1 but severe impairment of FEF 25–75 . Two cases showed a bronchodilator response in terms of FEV 1 (subjects 3 and 4), while 3 patients had a significant increase of FEF 25–75 (cases 1, 3 and 4). Unfortunately, we could not provide the results of bronchodilator response during or after the treatment with omalizumab in any case.
Available literature on the diagnostic approach to difficult asthma in children offers a number of reviews which basically summarize the steps needed to fill the gap between a generic diagnosis of “difficult asthma” and more specific labels (i.e., “severe” asthma, “difficult-to-treat” asthma, or even different diagnoses) [ 3 , 5 , 6 , 8 , 10 , 19 , 20 , 21 ]. So far, few original articles and case reports have been published, probably due to the peculiarity of the issue, which makes retrospective discussion of cases easier than the design of a prospective clinical study [ 4 , 22 , 23 , 24 , 25 , 26 ]. Available knowledge mainly derives from the experience of specialized centers.
The evaluation of a child referred for uncontrolled asthma should start with a careful history focused on typical respiratory symptoms and on the definition of possible triggers. In the “severe asthma” process, it is crucial for clinicians to maintain a high degree of skepticism about the ultimate diagnosis, particularly in the presence of relevant discrepancies between history, physical features and lung function, as many conditions may be misdiagnosed as asthma. In order to simplify this process, herein we propose an algorithm for the diagnosis of difficult-to-treat and severe asthma (Fig. 1 ). Confirmation of the diagnosis through a detailed clinical and laboratory re-evaluation is important because in 12–50% of cases assumed to have severe asthma this might not be the correct diagnosis [ 10 ]. Several documents have indicated the main steps of the process that should be followed in children with uncontrolled asthma [ 3 , 8 , 10 ]. The translation of these procedures into real life practice may deeply change from one subject to another due to the variability of individual patients’ history and clinical features, which will often lead the diagnostic investigations towards the most likely reason for uncontrolled asthma. For children with apparently severe asthma, the first step is to confirm the diagnosis and, before proceeding to broader investigations, to verify that the poor control is not simply determined by poor adherence to treatment, inadequate inhaler skills and/or environmental exposure to triggers. A nurse-led assessment, including a home visit, despite not being applicable in all settings, may be useful for identifying potentially modifiable factors in uncontrolled pediatric asthma [ 27 ].
A practical algorithm for the diagnosis of difficult-to-treat and severe asthma. ICS, inhaled corticosteroids; OCS, oral corticosteroids
A number of comorbidities have been increasingly recognized as factors that may impact asthma clinical expression and control in childhood [ 10 , 28 ]. Children with uncontrolled disease should be investigated for GER, rhinosinusitis, dysfunctional breathing and/or vocal cord dysfunction, obstructive sleep apnea, obesity, psychological factors, smoke exposure, hormonal influences, and ongoing drugs [ 3 , 6 , 8 , 20 ]. Indeed, the exact role played by comorbidities in pediatric asthma control is still debated [ 28 ]. The most impressive example is GER. Several pediatric documents recommend assessing for GER because reflux may be a contributing factor to problematic or difficult asthma [ 7 , 29 ]. Nevertheless, GER treatment might not be effective for severe asthma [ 30 , 31 ], as confirmed by current cases 1 and 2. There is an established evidence that chronic rhinosinusitis is associated with more severe asthma in children [ 32 , 33 , 34 ]. Therefore, examination of upper airways and ad hoc treatment if rhinosinusitis is evident are recommended in children with severe asthma [ 3 , 8 , 35 ]. However, intranasal steroids for rhinitis resulted in a small reduction of asthma risk in school-aged children [ 36 ], and actual placebo-controlled studies on the effect of treatment of rhinosinusitis on asthma control in children are lacking [ 10 , 37 ].
Dysfunctional breathing, including hyperventilation and vocal cord dysfunction, is associated with poorer asthma control in children [ 8 , 10 , 38 , 39 ]. Unfortunately, there is scarce literature on the effect of its treatment on the control of severe asthma in children [ 40 ]. SDB ranging from primary snoring to obstructive sleep apnea syndrome is very common in children [ 41 ], and an increased prevalence of SDB together with increasing asthma severity has been reported [ 42 ]. Interestingly, GER may also be worsened by recurrent episodes of upper airway obstruction associated with SDB, and this may further trigger bronchial obstruction. Asthma guidelines recommend the assessment of SDB through nocturnal polysomnography in poorly controlled asthmatics, particularly if they are also obese [ 5 ]. There are no studies examining whether pediatric asthma improves after SDB has been treated, for example, with nasal steroids, adenotonsillectomy, continuous positive airway pressure or weight reduction if the child is also obese [ 43 ]. The parallel increase in obesity and asthma suggests that the two conditions are linked and that they can aggravate each other [ 44 , 45 ], even though the exact mechanisms that underlie this association remain unclear [ 46 ]. Indeed, other coexisting comorbidities such as SDB or GER may play a confounding role in the development of the interactions between obesity and the airways [ 47 , 48 ]. Obesity is associated with increased markers of inflammation in serum and adipose tissue and yet decreased airway inflammation in obese people with asthma [ 49 ]. Several interventions, including behavioral and weight reduction programs or bariatric surgery, may result in improved asthma control, quality of life and lung function in adult obese asthmatics [ 50 ]. Although reports of adolescent bariatric surgery demonstrate a significant body weight decrease, this approach is not widely available and there are no published reports on its effect on pediatric severe asthma control [ 51 ]. Finally, although it is still unclear whether food allergy is causative or shares a common pathway with difficult asthma, it might explain the loss of asthma control at least in some children and thus be considered as a comorbid condition [ 10 , 16 , 52 ].
In conclusion, establishing the impact of comorbidities on asthma control may be cumbersome, and an ex-juvantibus treatment is sometimes necessary to assess their role. Comorbid conditions can also worsen each other, and symptoms arising from some of them may mimic asthma [ 6 ]. Although the ability to improve pediatric severe asthma by treating comorbidities remains unconfirmed, they should be treated appropriately [ 9 ].
The vast majority of asthmatic children exhibit a mild or at most a moderate disease that can be fully controlled with low-to-medium dose ICS associated or not with other controllers [ 5 , 6 ]. However, a subset of asthmatics remains difficult-to-treat [ 5 , 6 ]. With the advent of biologics, these severe steroid-dependent asthmatics have alternative options for treatment, as steroid-related adverse events are common in severe asthma [ 53 ]. Omalizumab, an anti-IgE monoclonal antibody, is the only biologic therapy recommended in children with moderate-to-severe asthma by the recent guidelines [ 5 , 6 ]. In Italy, this treatment is fully covered by the National Health System. Therefore, there is no influence by any funding on treatment decisions. It was approved by the US (Food and Drug Administration) in 2003 and by the European Union (European Medicines Agency) in 2005 as an add-on treatment for patients aged > 12 years with severe persistent allergic asthma and who have a positive skin test or in-vitro reactivity to a perennial aeroallergen, FEV 1 < 80% predicted, frequent daytime symptoms or nighttime awakenings, and multiple documented severe asthma exacerbations despite daily ICS plus a LABA [ 54 , 55 ]. In 2009, it also received approval in Europe for treating patients aged 6–12 years. Figure 2 illustrates current indications for treatment with omalizumab in children and adolescents with severe asthma.
Indications for omalizumab in children and adolescents with severe asthma
IgE antibodies, Th 2 -derived cytokines and eosinophils play a major role in the development of chronic airway inflammation in asthmatic subjects [ 56 ]. Once released from plasma cells, IgE binds principally to the high-affinity IgE receptor (FcεRI) on mast cells, triggering different effector responses, including the release of mediators leading to allergic inflammatory reactions [ 56 ]. The activation of the allergic cascade by IgE, under constant allergen stimulation, leads to the establishment of chronic allergic inflammation in the airways of asthmatic patients, with IgE being a key element of the vicious circle that maintains it. Cytokines produced during the late phase and subsequent chronic inflammation stage have been directly associated with the induction of airway remodelling, indirectly implicating IgE in the process [ 56 ]. At present, omalizumab is the only commercially available recombinant humanized anti-IgE monoclonal antibody that specifically binds serum free IgE at its CH 3 domain, in the proximity of the binding site for FcεRI, thus preventing IgE from interacting with its receptor on mast cells, basophils, antigen-presenting cells and other inflammatory cells [ 57 ]. The rapid reduction of free IgE levels leads to a downregulation of the FcεRI expression on inflammatory cells and an interruption of the allergic cascade, which results in the reduction of peripheral and bronchial tissue eosinophilia and of levels of granulocyte macrophage colony stimulating factor, interleukin (IL)-2, IL-4, IL-5, and IL-13 [ 58 ]. Moreover, basophils have a relevant role in the initiation and progression of allergic inflammation, suggesting that they may represent a viable therapeutic target. Indeed, in children with severe asthma, it has been reported that omalizumab therapy is associated with a significant reduction in circulating basophil numbers, a finding that is concurrent with improved clinical outcomes [ 59 ]. This finding supports a mechanistic link between IgE levels and circulating basophil populations, and may provide new insights into one mechanism by which omalizumab improves asthma symptoms.
Several clinical controlled and real-life studies of adults with severe, inadequately controlled allergic asthma have demonstrated the efficacy and safety of omalizumab in reducing asthma-related symptoms, corticosteroid use, exacerbation rates, and healthcare resource utilization, and in improving QoL and lung function [ 60 , 61 , 62 , 63 ]. Fewer studies have been published in children. In two double-blind, randomized, placebo-controlled trials (RCTs) of children aged 6 to 12 years with moderate-to-severe allergic asthma, treatment with omalizumab reduced the requirement for ICS and protected against disease exacerbations, but there was little change in asthma symptom scores or spirometry [ 9 , 64 ]. These findings were confirmed and extended in older children [ 65 , 66 , 67 ].
The results of the ICATA study, a multicenter RCT of 419 inner-city children, adolescents and young adults with persistent allergic asthma, showed that, compared to placebo, omalizumab reduces the number of days with asthma symptoms and the proportion of participants with at least one exacerbation by approximately 25% and 19%, respectively ( p < 0.001), thus reducing the need for asthmatic symptom controllers [ 68 ]. Another multicenter RCT of inner-city children and adolescents showed that the addition of omalizumab to ongoing guidelines-based care before patients return to school reduces fall asthma exacerbations (odds ratio, 0.48), particularly in subjects with a recent exacerbation [ 69 ]. Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases ( p < 0.001), while FEV 1 improved by 4.9% ( p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% ( p < 0.001) [ 70 ]. The same authors also showed that, after two years of treatment, exacerbation rate and healthcare utilisation were further decreased by 83% and 100%, respectively, while level of asthma control, steroid use and lung function remained unchanged [ 71 ].
A systematic review of pediatric RCTs pooled the data of 1381 children and adolescents with moderate-to-severe allergic asthma in order to establish the efficacy of omalizumab as an add-on therapy [ 72 ]. During the stable-steroid phase, omalizumab decreased the number of patients with at least one exacerbation (risk ratio, 0.69; p < 0.001), the mean number of asthma exacerbations per patient (risk ratio, 0.35; p < 0.001), and the asthma symptom score (mean difference, 0.12; p = 0.005) when compared to placebo. During the steroid reduction phase, omalizumab further reduced the number of patients with at least one exacerbation (risk ratio, 0.48; p < 0.001) and the mean number of asthma exacerbations per patient (mean difference, 0.12; p < 0.05).
Given the cost of omalizumab, many authors have argued for the importance of identifying specific asthma populations who will have significant benefit from it [ 68 , 73 , 74 ]. In the ICATA study, baseline predictors of good response to treatment were sensitization and exposure to cockroach allergen, sensitization to house dust mite allergens, a serum IgE level of more than 100 IU per milliliter, a BMI of 25 or more, and a history of at least one unscheduled medical visit in the previous year [ 68 ].
Several studies have assessed the long-term safety of omalizumab in children and adults. A pooled analysis of 67 RCTs conducted over 2 decades on 4254 children and adults treated with omalizumab showed no association between omalizumab treatment and risk of malignancy [ 75 ]. In an RCT evaluating 225 school-aged children, omalizumab was well tolerated, there were no serious adverse events, and the frequency and types of all adverse events were similar to the placebo group [ 9 ]. These results have been further confirmed by a recent systematic review of RCTs that concluded that treatment with omalizumab does not result in increased risk of malignancy or hypersensitivity reactions [ 72 ].
While the rationale for long-term treatment with omalizumab is supported by pharmacokinetic-pharmacodynamic models [ 76 ], the duration of treatment is still under discussion. Results from published studies suggest that omalizumab should be continued for > 1 year [ 77 , 78 ]. In a retrospective study of adults and children with uncontrolled severe asthma treated with omalizumab, the response to treatment was ‘excellent’ in 52.5% of patients, particularly in the subgroup of children aged 6 to 11 years [ 77 ]. After the discontinuation of treatment, loss of asthma control was documented in 69.2% of the patients who had received omalizumab for < 1 year, 59.1% of the subjects treated for 1–2 years, and 46.1% of the cases treated for > 2 years. Time to loss of control was shorter in younger children and longer in patients with an ‘excellent’ response compared with patients with a ‘good’ response. No early loss of control (within 6 months) was observed among patients with > 3.5 years of continuous treatment with omalizumab. Finally, 20% of patients in whom omalizumab was re-prescribed because of loss of control did not respond to the treatment anymore [ 77 ]. Despite these encouraging findings, the impact of omalizumab on the natural history of severe asthma in children deserves to be further investigated by long-term studies that will also define the criteria and timing for discontinuing the treatment.
It is well known that asthma pharmacotherapy is effective in controlling symptoms and bronchial inflammation, but cannot affect the underlying immune response, thus leading to the possibility of symptom reappearance after its discontinuation [ 79 ]. In this scenario, allergen-specific immunotherapy (AIT) has been proposed as the only therapeutic method that can modulate the underlying immune pathophysiology in allergic asthma [ 80 ].
AIT is currently indicated in children and adults with mild-moderate allergic asthma that is completely or partially controlled by pharmacotherapy and with the evidence of a clear relationship between symptoms and exposure to a specific allergen [ 81 , 82 , 83 , 84 ]. However, according to recent guidelines, the efficacy of AIT in asthmatic subjects is limited, and its potential benefits must be weighed against the risk of side effects and the inconvenience and costs of the prolonged therapy [ 5 ]. Moreover, severe or uncontrolled asthma (regardless of its severity) is a major independent risk factor for non-fatal or even fatal adverse reactions, thus representing a contraindication for AIT [ 85 , 86 , 87 ]. Finally, children with severe asthma are often sensitized to multiple allergens, thus making AIT prescription even more complicated [ 88 ].
In subjects with uncontrolled and/or severe allergic asthma, a combination of omalizumab and AIT has been proposed [ 88 ]. Surprisingly, only a few studies have addressed this issue [ 89 , 90 , 91 , 92 ]. However, pre-treatment with omalizumab seems to improve the efficacy and tolerability of subcutaneous AIT in children and adults with severe allergic asthma both during omalizumab treatment and after its discontinuation [ 89 , 91 , 92 ]. Omalizumab has also been successfully used as a supplementary treatment to AIT in order to improve asthma control in children ≥6 years with severe persistent allergic asthma [ 90 ]. Given the scarcity of studies on AIT plus omalizumab in children with severe allergic asthma, further research is warranted to assess risks and benefits of the combined treatment.
Children with severe asthma require a detailed and individualized approach including re-assessment for differential diagnoses, comorbidities and contributory factors, environmental triggers, lung function and inflammation, adherence and response to therapy, and QoL. Treatment of pediatric severe asthma still relies on the maximal optimal use of corticosteroids, bronchodilators and other controllers recommended for moderate-to-severe disease. However, the management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma.
In the current paper, we described the characteristics of four children with severe asthma in whom omalizumab was prescribed. A review of the relevant literature on the topic was also performed. Finally, we provided an algorithm for the diagnosis of difficult-to-treat and severe asthma in children and adolescents, based on the evidence from the literature review. As all algorithms, it is not meant to replace clinical judgment, but it should drive physicians to adopt a systematic approach towards difficult and severe asthma and provide a useful guide to the clinician.
The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism of outcome improvements in patients with allergic severe asthma. As severe asthma is a heterogeneous condition consisting of different phenotypes, the future of asthma management will likely involve phenotypic and potentially even genotypic characterization in selected cases in order to determine appropriate therapy and thus to provide the highest possible benefit, especially if specific responder phenotypes can be identified and selected for this highly specific treatment.
Abbreviations
Anti-immunoglobulin E
Body mass index
IgE receptor
Forced expiratory flow between 25% and 75%
Forced expiratory volume in the first second
Gastroesophageal reflux
Inhaled corticosteroids
Intensive care unit
Interleukin
Long-acting β 2 -agonist
Oral leukotriene receptor antagonist
Quality of life
Randomized controlled trials
Short-acting β 2 -agonists
Sleep-disordered breathing
O'Byrne PM, Pedersen S, Schatz M, Thoren A, Ekholm E, Carlsson LG, et al. The poorly explored impact of uncontrolled asthma. Chest. 2013;143:511–3.
Article PubMed Google Scholar
National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–8.
Article Google Scholar
Hedlin G. Management of severe asthma in childhood-state of the art and novel perspectives. Pediatr Allergy Immunol. 2014;25:111–21.
Konradsen JR, Nordlund B, Lidegran M, Pedroletti C, Grönlund H, van Hage M, et al. Problematic severe asthma: a proposed approach to identifying children who are severely resistant to therapy. Pediatr Allergy Immunol. 2011;22:9–18.
Global Initiative for Asthma Report. Global strategy for asthma management and prevention (updated 2016). https://www.ginasthma.org . Accessed 07 June 2017.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–53.
Article CAS PubMed Google Scholar
Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the north American Society for Pediatric Gastroenterology, Hepatology, and nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49:498–507.
Lødrup Carlsen KC, Hedlin G, Bush A, Wennergren G, de Benedictis FM, De Jongste JC, et al. Assessment of problematic severe asthma in children. Eur Respir J. 2011;37:432–40.
Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics. 2001;108:E36.
Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376:814–5.
Article CAS PubMed PubMed Central Google Scholar
Lang A, Mowinckel P, Sachs-Olsen C, Riiser A, Lunde J, Carlsen KH, et al. Asthma severity in childhood, untangling clinical phenotypes. Pediatr Allergy Immunol. 2010;21:945–53.
Nordlund B, Konradsen JR, Pedroletti C, Kull I, Hedlin G. The clinical benefit of evaluating health-related quality-of-life in children with problematic severe asthma. Acta Paediatr. 2011;100:1454–60.
Dean BB, Calimlim BC, Sacco P, Aguilar D, Maykut R, Tinkelman D. Uncontrolled asthma: assessing quality of life and productivity of children and their caregivers using a cross-sectional internet-based survey. Health Qual Life Outcomes. 2010;8:6.
Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46.
British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma, 2014. https://www.brit-thoracic.org.uk/guidelines-and-quality-standards/asthma-guideline . Accessed 13 Apr 2016.
Montella S, Baraldi E, Cazzato S, Aralla R, Berardi M, Brunetti LM, et al. Severe asthma features in children: a case-control online survey. Ital J Pediatr. 2016;42:9.
Article PubMed PubMed Central Google Scholar
Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG, National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–25.
Simon MR, Chinchilli VM, Phillips BR, Sorkness CA, Lemanske RF Jr, Szefler SJ, et al. Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010;126:527–34.
Hedlin G, Bush A, Lødrup Carlsen K, Wennergren G, De Benedictis FM, Melén E, et al. Problematic severe asthma in children, not one problem but many: a GA2LEN initiative. Eur Respir J. 2010;36:196–201.
Fitzpatrick AM, Teague WG. Severe asthma in children: insights from the National Heart, Lung, and Blood Institute's severe asthma research program. Pediatr Allergy Immunol Pulmonol. 2010;23:131–8.
Konradsen JR, Caffrey Osvald E, Hedlin G. Update on the current methods for the diagnosis and treatment of severe childhood asthma. Expert Rev Respir Med. 2015;9:769–77.
Lang AM, Konradsen J, Carlsen KH, Sachs-Olsen C, Mowinckel P, Hedlin G, et al. Identifying problematic severe asthma in the individual child—does lung function matter? Acta Paediatr. 2010;99:404–10.
Rao DR, Gaffin JM, Baxi SN, Sheehan WJ, Hoffman EB, Phipatanakul WJ. The utility of forced expiratory flow between 25% and 75% of vital capacity in predicting childhood asthma morbidity and severity. Asthma. 2012;49:586–92.
Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics. 2000;105:354–8.
Cicutto LC, Chapman KR, Chamberlain D, Downey GP. Difficult asthma: consider all of the possibilities. Can Respir J. 2000;7:415–8.
Wener RR, Bel EH. Severe refractory asthma: an update. Eur Respir Rev. 2013;22:227–35.
Bracken M, Fleming L, Hall P, et al. The importance of nurse-led home visits in the assessment of children with problematic asthma. Arch Dis Child. 2009;94:780–4.
De Groot EP, Kreggemeijer WJ, Brand PL. Getting the basics right resolves most cases of uncontrolled and problematic asthma. Acta Paediatr. 2015;104:916–21.
Grimaldi-Bensouda L, Zureik M, Aubier M, Humbert M, Levy J, Benichou J, et al. Does omalizumab make a difference to the real-life treatment of asthma exacerbations? Results from a large cohort of patients with severe uncontrolled asthma. Chest. 2013;143:398–405.
American Lung Association Asthma Clinical Research Centers, Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487–9.
Article PubMed Central Google Scholar
Writing Committee for the American Lung Association Asthma Clinical Research Centers, Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA 2012;307:373-381.
Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94:895–901.
CAS PubMed Google Scholar
De Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67:582–7.
Rotiroti G, Roberts G, Scadding GK. Rhinitis in children: common clinical presentations and differential diagnoses. Pediatr Allergy Immunol. 2015;26:103–10.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA). 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63:S8–160.
Deliu M, Belgrave D, Simpson A, Murray CS, Kerry G, Custovic A. Impact of rhinitis on asthma severity in school-age children. Allergy. 2014;69:1515–21.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–76.
Weinberger M, Abu-Hasan M. Pseudo-asthma: when cough, wheezing, and dyspnea are not asthma. Pediatrics. 2007;120:855–64.
De Groot EP, Duiverman EJ, Brand PL. Dysfunctional breathing in children with asthma: a rare but relevant comorbidity. Eur Respir J. 2013;41:1068–73.
Barker NJ, Jones M, O'Connell NE, Everard ML. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in children. Cochrane Database Syst Rev. 2013;12:CD010376.
Google Scholar
Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12.
Goldstein NA, Aronin C, Kantrowitz B, Hershcopf R, Fishkin S, Lee H, Weaver DE, et al. The prevalence of sleep-disordered breathing in children with asthma and its behavioral effects. Pediatr Pulmonol. 2015;50:1128–36.
Ross KR, Storfer-Isser A, Hart MA, Kibler AM, Rueschman M, Rosen CL, et al. Sleep-disordered breathing is associated with asthma severity in children. J Pediatr. 2012;160:736–42.
Santamaria F, Montella S, Greco L, Valerio G, Franzese A, Maniscalco M, et al. Obesity duration is associated to pulmonary function impairment in obese subjects. Obesity (Silver Spring). 2011;19:1623–8.
Sivapalan P, Diamant Z, Ulrik CS. Obesity and asthma: current knowledge and future needs. Curr Opin Pulm Med. 2015;21:80–5.
Rasmussen F, Hancox RJ. Mechanisms of obesity in asthma. Curr Opin Allergy Clin Immunol. 2014;14:35–43.
Santamaria F, Montella S, Pietrobelli A. Obesity and pulmonary disease: unanswered questions. Obes Rev. 2012;13:822–33.
Lang JE, Hossain J, Holbrook JT, Teague WG, Gold BD, Wise RA, et al. Gastro-oesophageal reflux and worse asthma control in obese children: a case of symptom misattribution? Thorax. 2016;71:238–46.
Santamaria F, Montella S, De Stefano S, Sperlì F, Barbarano F, Valerio G. Relationship between exhaled nitric oxide and body mass index in children and adolescents. J Allergy Clin Immunol. 2005;116:1163–4.
Van Huisstede A, Rudolphus A, Castro Cabezas M, Biter LU, van de Geijn GJ, Taube C, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70:659–67.
Katzmarzyk PT, Bouchard C. Where is the beef? Waist circumference is more highly correlated with BMI and total body fat than with abdominal visceral fat in children. Int J Obes. 2014;38:753–4.
Article CAS Google Scholar
De Groot EP, Duiverman EJ, Brand PL. Comorbidities of asthma during childhood: possibly important, yet poorly studied. Eur Respir J. 2010;36:671–8.
Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016; https://doi.org/10.1136/thoraxjnl-2015-207630 .
Federal Drug Administration Advisory for Omalizumab. Available at: https://wayback.archive-it.org/7993/20170111075347/ . http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/default.htm . Accessed 4 Feb 2018.
European Medicines Agency: assessment report for Xolair. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000606/human_med_001162.jsp&mid=WC0b01ac058001d124 . Accessed 7 June 2017.
Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086–96.
Jensen RK, Plum M, Tjerrild L, Jakob T, Spillner E, Andersen GR. Structure of the omalizumab Fab. Acta Crystallogr F Struct Biol Commun. 2015;71:419–26.
Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64:1728–36.
Hill DA, Siracusa MC, Ruymann KR, Tait Wojno ED, Artis D, Spergel JM. Omalizumab therapy is associated with reduced circulating basophil populations in asthmatic children. Allergy. 2014;69:674–7.
Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16.
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
Lai T, Wang S, Xu Z, Zhang C, Zhao Y, Hu Y, Cao C, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015;5:8191.
Abraham I, Alhossan A, Lee CS, Kutbi H, MacDonald K. “real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2015; https://doi.org/10.1111/all.12815 .
Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124:1210–6.
Solèr M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–61.
Holgate ST. Cytokine and anti-cytokine therapy for the treatment of asthma and allergic disease. Cytokine. 2004;28:152–7.
Odajima H, Ebisawa M, Nagakura T, Fujisawa T, Akasawa A, Ito K, et al. Omalizumab in Japanese children with severe allergic asthma uncontrolled with standard therapy. Allergol Int. 2015;64:364–70.
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15.
Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–85.
Deschildre A, Marguet C, Salleron J, Pin I, Rittié JL, Derelle J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013;42:1224–33.
Deschildre A, Marguet C, Langlois C, Pin I, Rittié JL, Derelle J, et al. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur Respir J. 2015;46:856–9.
Rodrigo GJ, Neffen H. Systematic review on the use of omalizumab for the treatment of asthmatic children and adolescents. Pediatr Allergy Immunol. 2015;26:551–6.
Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2004;114:265–9.
Campbell JD, Spackman DE, Sullivan SD. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy. 2010;65:1141–8.
Busse W, Buhl R, Fernandez Vidaurre C, Blogg M, Zhu J, Eisner MD, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129:983–9.
Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72:306–10.
Molimard M, Mala L, Bourdeix I, Le Gros V. Observational study in severe asthmatic patients after discontinuation of omalizumab for good asthma control. Respir Med. 2014;108:571–6.
Busse WW, Trzaskoma B, Omachi TA, Canvin J, Rosen K, Chipps BE, et al. Evaluating Xolair persistency of response after long-term therapy (XPORT). Am J Respir Crit Care Med. 2014;189:A6576.
Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97.
Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012;18:736–49.
National Heart, Lung, and Blood Institute. Expert panel report 3: Guidelines for the diagnosis and management of asthma—full report 2007. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf . Accessed 4 Feb 2018.
Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, Joint Council of Allergy, Asthma and Immunolgy. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25–85.
Zuberbier T, Bachert C, Bousquet PJ, Passalacqua G, Walter Canonica G, Merk H, et al. GA(2) LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–30.
Pajno GB, Bernardini R, Peroni D, Arasi S, Martelli A, Landi M, et al. Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Ital J Pediatr. 2017;43:13.
Pitsios C, Demoly P, Bilo MB, Gerth van Wijk R, Pfaar O, Sturm GJ, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70:897–909.
Tsabouri S, Mavroudi A, Feketea G, Guibas GV. Subcutaneous and sublingual immunotherapy in allergic asthma in children. Front Pediatr. 2017;5:82.
Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–68.
Hedlin G, van Hage M. The role of immunotherapy in the management of childhood asthma. Ther Adv Respir Dis. 2012;6:137–46.
Lambert N, Guiddir T, Amat F, Just J. Pre-treatment by omalizumab allows allergen immunotherapy in children and young adults with severe allergic asthma. Pediatr Allergy Immunol. 2014;25:829–32.
Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann K-C, Sieder C. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy. 2009;39:271–9.
Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125:383–9.
Stelmach I, Kaczmarek-Woźniak J, Majak P, Olszowiec-Chlebna M, Jerzynska J. Efficacy and safety of high-doses sublingual immunotherapy in ultra-rush scheme in children allergic to grass pollen. Clin Exp Allergy. 2009;39:401–8.
Download references
Acknowledgements
The authors gratefully thank Dr. Marco Maglione for his contribution in the clinical assessment of the described cases. Medical writing assistance was provided by Stephen Walters on behalf of City Hills Proofreading.
No funding was secured for this study.
Availability of data and materials
All relevant data and materials are published in the manuscript.
Author information
Authors and affiliations.
Department of Translational Medical Sciences, Federico II University, Via Sergio Pansini 5, 80131, Naples, Italy
Virginia Mirra, Silvia Montella & Francesca Santamaria
You can also search for this author in PubMed Google Scholar
Contributions
VM, SM and FS, authors of the current manuscript, declare that they have participated sufficiently in the work to take public responsibility for appropriate portions of the content. VM and SM carried out the initial investigations, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted. FS conceptualized and designed the study, and critically reviewed and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Francesca Santamaria .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethics committee “Carlo Romano”, Federico II University, Naples, Italy. Children’s parents/legal guardians gave informed written consent to participate. The description of our cases adheres to the CARE standards of reporting checklist.
Consent for publication
Children’s parents/legal guardians provided informed written consent for the case report to be published.
Competing interests
The authors declare that they have no competing interests to disclose. Authors have no financial relationships relevant to this article to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Mirra, V., Montella, S. & Santamaria, F. Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment. BMC Pediatr 18 , 73 (2018). https://doi.org/10.1186/s12887-018-1019-9
Download citation
Received : 24 May 2016
Accepted : 29 January 2018
Published : 21 February 2018
DOI : https://doi.org/10.1186/s12887-018-1019-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Severe asthma
- Adolescents
- Asthma exacerbations
BMC Pediatrics
ISSN: 1471-2431
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Open access
- Published: 16 October 2014
A woman with asthma: a whole systems approach to supporting self-management
- Hilary Pinnock 1 ,
- Elisabeth Ehrlich 1 ,
- Gaylor Hoskins 2 &
- Ron Tomlins 3
npj Primary Care Respiratory Medicine volume 24 , Article number: 14063 ( 2014 ) Cite this article
16k Accesses
2 Citations
6 Altmetric
Metrics details
- Health care
A 35-year-old lady attends for review of her asthma following an acute exacerbation. There is an extensive evidence base for supported self-management for people living with asthma, and international and national guidelines emphasise the importance of providing a written asthma action plan. Effective implementation of this recommendation for the lady in this case study is considered from the perspective of a patient, healthcare professional, and the organisation. The patient emphasises the importance of developing a partnership based on honesty and trust, the need for adherence to monitoring and regular treatment, and involvement of family support. The professional considers the provision of asthma self-management in the context of a structured review, with a focus on a self-management discussion which elicits the patient’s goals and preferences. The organisation has a crucial role in promoting, enabling and providing resources to support professionals to provide self-management. The patient’s asthma control was assessed and management optimised in two structured reviews. Her goal was to avoid disruption to her work and her personalised action plan focused on achieving that goal.
Similar content being viewed by others
Barriers to implementing asthma self-management in Malaysian primary care: qualitative study exploring the perspectives of healthcare professionals

The self-management abilities test (SMAT): a tool to identify the self-management abilities of adults with bronchiectasis
Improving primary care management of asthma: do we know what really works?
A 35-year-old sales representative attends the practice for an asthma review. Her medical record notes that she has had asthma since childhood, and although for many months of the year her asthma is well controlled (when she often reduces or stops her inhaled steroids), she experiences one or two exacerbations a year requiring oral steroids. These are usually triggered by a viral upper respiratory infection, though last summer when the pollen count was particularly high she became tight chested and wheezy for a couple of weeks.
Her regular prescription is for fluticasone 100 mcg twice a day, and salbutamol as required. She has a young family and a busy lifestyle so does not often manage to find time to attend the asthma clinic. A few weeks previously, an asthma attack had interfered with some important work-related travel, and she has attended the clinic on this occasion to ask about how this can be managed better in the future. There is no record of her having been given an asthma action plan.
What do we know about asthma self-management? The academic perspective
Supported self-management reduces asthma morbidity.
The lady in this case study is struggling to maintain control of her asthma within the context of her busy professional and domestic life. The recent unfortunate experience which triggered this consultation offers a rare opportunity to engage with her and discuss how she can manage her asthma better. It behoves the clinician whom she is seeing (regardless of whether this is in a dedicated asthma clinic or an appointment in a routine general practice surgery) to grasp the opportunity and discuss self-management and provide her with a (written) personalised asthma action plan (PAAP).
The healthcare professional advising the lady is likely to be aware that international and national guidelines emphasise the importance of supporting self-management. 1 – 4 There is an extensive evidence base for asthma self-management: a recent synthesis identified 22 systematic reviews summarising data from 260 randomised controlled trials encompassing a broad range of demographic, clinical and healthcare contexts, which concluded that asthma self-management reduces emergency use of healthcare resources, including emergency department visits, hospital admissions and unscheduled consultations and improves markers of asthma control, including reduced symptoms and days off work, and improves quality of life. 1 , 2 , 5 – 12 Health economic analysis suggests that it is not only clinically effective, but also a cost-effective intervention. 13
Personalised asthma action plans
Key features of effective self-management approaches are:
Self-management education should be reinforced by provision of a (written) PAAP which reminds patients of their regular treatment, how to monitor and recognise that control is deteriorating and the action they should take. 14 – 16 As an adult, our patient can choose whether she wishes to monitor her control with symptoms or by recording peak flows (or a combination of both). 6 , 8 , 9 , 14 Symptom-based monitoring is generally better in children. 15 , 16
Plans should have between two and three action points including emergency doses of reliever medication; increasing low dose (or recommencing) inhaled steroids; or starting a course of oral steroids according to severity of the exacerbation. 14
Personalisation of the action plan is crucial. Focussing specifically on what actions she could take to prevent a repetition of the recent attack is likely to engage her interest. Not all patients will wish to start oral steroids without advice from a healthcare professional, though with her busy lifestyle and travel our patient is likely to be keen to have an emergency supply of prednisolone. Mobile technology has the potential to support self-management, 17 , 18 though a recent systematic review concluded that none of the currently available smart phone ‘apps’ were fit for purpose. 19
Identification and avoidance of her triggers is important. As pollen seems to be a trigger, management of allergic rhinitis needs to be discussed (and included in her action plan): she may benefit from regular use of a nasal steroid spray during the season. 20
Self-management as recommended by guidelines, 1 , 2 focuses narrowly on adherence to medication/monitoring and the early recognition/remediation of exacerbations, summarised in (written) PAAPs. Patients, however, may want to discuss how to reduce the impact of asthma on their life more generally, 21 including non-pharmacological approaches.
Supported self-management
The impact is greater if self-management education is delivered within a comprehensive programme of accessible, proactive asthma care, 22 and needs to be supported by ongoing regular review. 6 With her busy lifestyle, our patient may be reluctant to attend follow-up appointments, and once her asthma is controlled it may be possible to make convenient arrangements for professional review perhaps by telephone, 23 , 24 or e-mail. Flexible access to professional advice (e.g., utilising diverse modes of consultation) is an important component of supporting self-management. 25
The challenge of implementation
Implementation of self-management, however, remains poor in routine clinical practice. A recent Asthma UK web-survey estimated that only 24% of people with asthma in the UK currently have a PAAP, 26 with similar figures from Sweden 27 and Australia. 28 The general practitioner may feel that they do not have time to discuss self-management in a routine surgery appointment, or may not have a supply of paper-based PAAPs readily available. 29 However, as our patient rarely finds time to attend the practice, inviting her to make an appointment for a future clinic is likely to be unsuccessful and the opportunity to provide the help she needs will be missed.
The solution will need a whole systems approach
A systematic meta-review of implementing supported self-management in long-term conditions (including asthma) concluded that effective implementation was multifaceted and multidisciplinary; engaging patients, training and motivating professionals within the context of an organisation which actively supported self-management. 5 This whole systems approach considers that although patient education, professional training and organisational support are all essential components of successful support, they are rarely effective in isolation. 30 A systematic review of interventions that promote provision/use of PAAPs highlighted the importance of organisational systems (e.g., sending blank PAAPs with recall reminders). 31 A patient offers her perspective ( Box 1 ), a healthcare professional considers the clinical challenge, and the challenges are discussed from an organisational perspective.
Box 1: What self-management help should this lady expect from her general practitioner or asthma nurse? The patient’s perspective
The first priority is that the patient is reassured that her condition can be managed successfully both in the short and the long term. A good working relationship with the health professional is essential to achieve this outcome. Developing trust between patient and healthcare professional is more likely to lead to the patient following the PAAP on a long-term basis.
A review of all medication and possible alternative treatments should be discussed. The patient needs to understand why any changes are being made and when she can expect to see improvements in her condition. Be honest, as sometimes it will be necessary to adjust dosages before benefits are experienced. Be positive. ‘There are a number of things we can do to try to reduce the impact of asthma on your daily life’. ‘Preventer treatment can protect against the effect of pollen in the hay fever season’. If possible, the same healthcare professional should see the patient at all follow-up appointments as this builds trust and a feeling of working together to achieve the aim of better self-management.
Is the healthcare professional sure that the patient knows how to take her medication and that it is taken at the same time each day? The patient needs to understand the benefit of such a routine. Medication taken regularly at the same time each day is part of any self-management regime. If the patient is unused to taking medication at the same time each day then keeping a record on paper or with an electronic device could help. Possibly the patient could be encouraged to set up a system of reminders by text or smartphone.
Some people find having a peak flow meter useful. Knowing one's usual reading means that any fall can act as an early warning to put the PAAP into action. Patients need to be proactive here and take responsibility.
Ongoing support is essential for this patient to ensure that she takes her medication appropriately. Someone needs to be available to answer questions and provide encouragement. This could be a doctor or a nurse or a pharmacist. Again, this is an example of the partnership needed to achieve good asthma control.
It would also be useful at a future appointment to discuss the patient’s lifestyle and work with her to reduce her stress. Feeling better would allow her to take simple steps such as taking exercise. It would also be helpful if all members of her family understood how to help her. Even young children can do this.
From personal experience some people know how beneficial it is to feel they are in a partnership with their local practice and pharmacy. Being proactive produces dividends in asthma control.
What are the clinical challenges for the healthcare professional in providing self-management support?
Due to the variable nature of asthma, a long-standing history may mean that the frequency and severity of symptoms, as well as what triggers them, may have changed over time. 32 Exacerbations requiring oral steroids, interrupting periods of ‘stability’, indicate the need for re-assessment of the patient’s clinical as well as educational needs. The patient’s perception of stability may be at odds with the clinical definition 1 , 33 —a check on the number of short-acting bronchodilator inhalers the patient has used over a specific period of time is a good indication of control. 34 Assessment of asthma control should be carried out using objective tools such as the Asthma Control Test or the Royal College of Physicians three questions. 35 , 36 However, it is important to remember that these assessment tools are not an end in themselves but should be a springboard for further discussion on the nature and pattern of symptoms. Balancing work with family can often make it difficult to find the time to attend a review of asthma particularly when the patient feels well. The practice should consider utilising other means of communication to maintain contact with patients, encouraging them to come in when a problem is highlighted. 37 , 38 Asthma guidelines advocate a structured approach to ensure the patient is reviewed regularly and recommend a detailed assessment to enable development of an appropriate patient-centred (self)management strategy. 1 – 4
Although self-management plans have been shown to be successful for reducing the impact of asthma, 21 , 39 the complexity of managing such a fluctuating disease on a day-to-day basis is challenging. During an asthma review, there is an opportunity to work with the patient to try to identify what triggers their symptoms and any actions that may help improve or maintain control. 38 An integral part of personalised self-management education is the written PAAP, which gives the patient the knowledge to respond to the changes in symptoms and ensures they maintain control of their asthma within predetermined parameters. 9 , 40 The PAAP should include details on how to monitor asthma, recognise symptoms, how to alter medication and what to do if the symptoms do not improve. The plan should include details on the treatment to be taken when asthma is well controlled, and how to adjust it when the symptoms are mild, moderate or severe. These action plans need to be developed between the doctor, nurse or asthma educator and the patient during the review and should be frequently reviewed and updated in partnership (see Box 1). Patient preference as well as clinical features such as whether she under- or over-perceives her symptoms should be taken into account when deciding whether the action plan is peak flow or symptom-driven. Our patient has a lot to gain from having an action plan. She has poorly controlled asthma and her lifestyle means that she will probably see different doctors (depending who is available) when she needs help. Being empowered to self-manage could make a big difference to her asthma control and the impact it has on her life.
The practice should have protocols in place, underpinned by specific training to support asthma self-management. As well as ensuring that healthcare professionals have appropriate skills, this should include training for reception staff so that they know what action to take if a patient telephones to say they are having an asthma attack.
However, focusing solely on symptom management strategies (actions) to follow in the presence of deteriorating symptoms fails to incorporate the patients’ wider views of asthma, its management within the context of her/his life, and their personal asthma management strategies. 41 This may result in a failure to use plans to maximise their health potential. 21 , 42 A self-management strategy leading to improved outcomes requires a high level of patient self-efficacy, 43 a meaningful partnership between the patient and the supporting health professional, 42 , 44 and a focused self-management discussion. 14
Central to both the effectiveness and personalisation of action plans, 43 , 45 in particular the likelihood that the plan will lead to changes in patients’ day-to-day self-management behaviours, 45 is the identification of goals. Goals are more likely to be achieved when they are specific, important to patients, collaboratively set and there is a belief that these can be achieved. Success depends on motivation 44 , 46 to engage in a specific behaviour to achieve a valued outcome (goal) and the ability to translate the behavioural intention into action. 47 Action and coping planning increases the likelihood that patient behaviour will actually change. 44 , 46 , 47 Our patient has a goal: she wants to avoid having her work disrupted by her asthma. Her personalised action plan needs to explicitly focus on achieving that goal.
As providers of self-management support, health professionals must work with patients to identify goals (valued outcomes) that are important to patients, that may be achievable and with which they can engage. The identification of specific, personalised goals and associated feasible behaviours is a prerequisite for the creation of asthma self-management plans. Divergent perceptions of asthma and how to manage it, and a mismatch between what patients want/need from these plans and what is provided by professionals are barriers to success. 41 , 42
What are the challenges for the healthcare organisation in providing self-management support?
A number of studies have demonstrated the challenges for primary care physicians in providing ongoing support for people with asthma. 31 , 48 , 49 In some countries, nurses and other allied health professionals have been trained as asthma educators and monitor people with stable asthma. These resources are not always available. In addition, some primary care services are delivered in constrained systems where only a few minutes are available to the practitioner in a consultation, or where only a limited range of asthma medicines are available or affordable. 50
There is recognition that the delivery of quality care depends on the competence of the doctor (and supporting health professionals), the relationship between the care providers and care recipients, and the quality of the environment in which care is delivered. 51 This includes societal expectations, health literacy and financial drivers.
In 2001, the Australian Government adopted a programme developed by the General Practitioner Asthma Group of the National Asthma Council Australia that provided a structured approach to the implementation of asthma management guidelines in a primary care setting. 52 Patients with moderate-to-severe asthma were eligible to participate. The 3+ visit plan required confirmation of asthma diagnosis, spirometry if appropriate, assessment of trigger factors, consideration of medication and patient self-management education including provision of a written PAAP. These elements, including regular medical review, were delivered over three visits. Evaluation demonstrated that the programme was beneficial but that it was difficult to complete the third visit in the programme. 53 – 55 Accordingly, the programme, renamed the Asthma Cycle of Care, was modified to incorporate two visits. 56 Financial incentives are provided to practices for each patient who receives this service each year.
Concurrently, other programmes were implemented which support practice-based care. Since 2002, the National Asthma Council has provided best-practice asthma and respiratory management education to health professionals, 57 and this programme will be continuing to 2017. The general practitioner and allied health professional trainers travel the country to provide asthma and COPD updates to groups of doctors, nurses and community pharmacists. A number of online modules are also provided. The PACE (Physician Asthma Care Education) programme developed by Noreen Clark has also been adapted to the Australian healthcare system. 58 In addition, a pharmacy-based intervention has been trialled and implemented. 59
To support these programmes, the National Asthma Council ( www.nationalasthma.org.au ) has developed resources for use in practices. A strong emphasis has been on the availability of a range of PAAPs (including plans for using adjustable maintenance dosing with ICS/LABA combination inhalers), plans for indigenous Australians, paediatric plans and plans translated into nine languages. PAAPs embedded in practice computer systems are readily available in consultations, and there are easily accessible online paediatric PAAPs ( http://digitalmedia.sahealth.sa.gov.au/public/asthma/ ). A software package, developed in the UK, can be downloaded and used to generate a pictorial PAAP within the consultation. 60
One of the strongest drivers towards the provision of written asthma action plans in Australia has been the Asthma Friendly Schools programme. 61 , 62 Established with Australian Government funding and the co-operation of Education Departments of each state, the Asthma Friendly Schools programme engages schools to address and satisfy a set of criteria that establishes an asthma-friendly environment. As part of accreditation, the school requires that each child with asthma should have a written PAAP prepared by their doctor to assist (trained) staff in managing a child with asthma at school.
The case study continues...
The initial presentation some weeks ago was during an exacerbation of asthma, which may not be the best time to educate a patient. It is, however, a splendid time to build on their motivation to feel better. She agreed to return after her asthma had settled to look more closely at her asthma control, and an appointment was made for a routine review.
At this follow-up consultation, the patient’s diagnosis was reviewed and confirmed and her trigger factors discussed. For this lady, respiratory tract infections are the usual trigger but allergic factors during times of high pollen count may also be relevant. Assessment of her nasal airway suggested that she would benefit from better control of allergic rhinitis. Other factors were discussed, as many patients are unaware that changes in air temperature, exercise and pets can also trigger asthma exacerbations. In addition, use of the Asthma Control Test was useful as an objective assessment of control as well as helping her realise what her life could be like! Many people with long-term asthma live their life within the constraints of their illness, accepting that is all that they can do.
After assessing the level of asthma control, a discussion about management options—trigger avoidance, exercise and medicines—led to the development of a written PAAP. Asthma can affect the whole family, and ways were explored that could help her family understand why it is important that she finds time in the busy domestic schedules to take her regular medication. Family and friends can also help by understanding what triggers her asthma so that they can avoid exposing her to perfumes, pollens or pets that risk triggering her symptoms. Information from the national patient organisation was provided to reinforce the messages.
The patient agreed to return in a couple of weeks, and a recall reminder was set up. At the second consultation, the level of control since the last visit will be explored including repeat spirometry, if appropriate. Further education about the pathophysiology of asthma and how to recognise early warning signs of loss of control can be given. Device use will be reassessed and the PAAP reviewed. Our patient’s goal is to avoid disruption to her work and her PAAP will focus on achieving that goal. Finally, agreement will be reached with the patient about future routine reviews, which, now that she has a written PAAP, could be scheduled by telephone if all is well, or face-to-face if a change in her clinical condition necessitates a more comprehensive review.
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2012. Available from: http://www.ginasthma.org (accessed July 2013).
British Thoracic Society/Scottish Intercollegiate Guideline Network British Guideline on the Management of Asthma. Thorax 2008; 63 (Suppl 4 iv1–121, updated version available from: http://www.sign.ac.uk (accessed January 2014).
Article Google Scholar
National Asthma Council Australia. Australian Asthma Handbook. Available from: http://www.nationalasthma.org.au/handbook (accessed May 2014).
National Asthma Education and Prevention Program (NAEPP) Coordinating Committee. Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma. Available from: https://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm (accessed May 2014).
Taylor SJC, Pinnock H, Epiphaniou E, Pearce G, Parke H . A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions. (PRISMS Practical Systematic Review of Self-Management Support for long-term conditions). Health Serv Deliv Res (in press).
Gibson PG, Powell H, Wilson A, Abramson MJ, Haywood P, Bauman A et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2002: (Issue 3) Art No. CD001117.
Tapp S, Lasserson TJ, Rowe BH . Education interventions for adults who attend the emergency room for acute asthma. Cochrane Database Syst Rev 2007: (Issue 3) Art No. CD003000.
Powell H, Gibson PG . Options for self-management education for adults with asthma. Cochrane Database Syst Rev 2002: (Issue 3) Art No: CD004107.
Toelle B, Ram FSF . Written individualised management plans for asthma in children and adults. Cochrane Database Syst Rev 2004: (Issue 1) Art No. CD002171.
Lefevre F, Piper M, Weiss K, Mark D, Clark N, Aronson N . Do written action plans improve patient outcomes in asthma? An evidence-based analysis. J Fam Pract 2002; 51 : 842–848.
PubMed Google Scholar
Boyd M, Lasserson TJ, McKean MC, Gibson PG, Ducharme FM, Haby M . Interventions for educating children who are at risk of asthma-related emergency department attendance. Cochrane Database Syst Rev 2009: (Issue 2) Art No.CD001290.
Bravata DM, Gienger AL, Holty JE, Sundaram V, Khazeni N, Wise PH et al. Quality improvement strategies for children with asthma: a systematic review. Arch Pediatr Adolesc Med 2009; 163 : 572–581.
Bower P, Murray E, Kennedy A, Newman S, Richardson G, Rogers A . Self-management support interventions to reduce health care utilisation without compromising outcomes: a rapid synthesis of the evidence. Available from: http://www.nets.nihr.ac.uk/projects/hsdr/11101406 (accessed April 2014).
Gibson PG, Powell H . Written action plans for asthma: an evidence-based review of the key components. Thorax 2004; 59 : 94–99.
Article CAS Google Scholar
Bhogal SK, Zemek RL, Ducharme F . Written action plans for asthma in children. Cochrane Database Syst Rev 2006: (Issue 3) Art No. CD005306.
Zemek RL, Bhogal SK, Ducharme FM . Systematic review of randomized controlled trials examining written action plans in children: what is the plan?. Arch Pediatr Adolesc Med 2008; 162 : 157–163.
Pinnock H, Slack R, Pagliari C, Price D, Sheikh A . Understanding the potential role of mobile phone based monitoring on asthma self-management: qualitative study. Clin Exp Allergy 2007; 37 : 794–802.
de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, Atun R . Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev 2012: (Issue 12) Art No. CD007459.
Huckvale K, Car M, Morrison C, Car J . Apps for asthma self-management: a systematic assessment of content and tools. BMC Med 2012; 10 : 144.
Allergic Rhinitis and its Impact on Asthma. Management of Allergic Rhinitis and its Impact on Asthma: Pocket Guide. ARIA 2008. Available from: http://www.whiar.org (accessed May 2014).
Ring N, Jepson R, Hoskins G, Wilson C, Pinnock H, Sheikh A et al. Understanding what helps or hinders asthma action plan use: a systematic review and synthesis of the qualitative literature. Patient Educ Couns 2011; 85 : e131–e143.
Moullec G, Gour-Provencal G, Bacon SL, Campbell TS, Lavoie KL . Efficacy of interventions to improve adherence to inhaled corticosteroids in adult asthmatics: Impact of using components of the chronic care model. Respir Med 2012; 106 : 1211–1225.
Pinnock H, Bawden R, Proctor S, Wolfe S, Scullion J, Price D et al. Accessibility, acceptability and effectiveness of telephone reviews for asthma in primary care: randomised controlled trial. BMJ 2003; 326 : 477–479.
Pinnock H, Adlem L, Gaskin S, Harris J, Snellgrove C, Sheikh A . Accessibility, clinical effectiveness and practice costs of providing a telephone option for routine asthma reviews: phase IV controlled implementation study. Br J Gen Pract 2007; 57 : 714–722.
PubMed PubMed Central Google Scholar
Kielmann T, Huby G, Powell A, Sheikh A, Price D, Williams S et al. From support to boundary: a qualitative study of the border between self care and professional care. Patient Educ Couns 2010; 79 : 55–61.
Asthma UK . Compare your care report. Asthma UK, 2013. Available from: http://www.asthma.org.uk (accessed January 2014).
Stallberg B, Lisspers K, Hasselgren M, Janson C, Johansson G, Svardsudd K . Asthma control in primary care in Sweden: a comparison between 2001 and 2005. Prim Care Respir J 2009; 18 : 279–286.
Reddel H, Peters M, Everett P, Flood P, Sawyer S . Ownership of written asthma action plans in a large Australian survey. Eur Respir J 2013; 42 . Abstract 2011.
Wiener-Ogilvie S, Pinnock H, Huby G, Sheikh A, Partridge MR, Gillies J . Do practices comply with key recommendations of the British Asthma Guideline? If not, why not? Prim Care Respir J 2007; 16 : 369–377.
Kennedy A, Rogers A, Bower P . Support for self care for patients with chronic disease. BMJ 2007; 335 : 968–970.
Ring N, Malcolm C, Wyke S, Macgillivray S, Dixon D, Hoskins G et al. Promoting the Use of Personal Asthma Action Plans: A Systematic Review. Prim Care Respir J 2007; 16 : 271–283.
Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB et al. A new perspective on concepts of asthma severity and control. Eur Respir J 2008; 32 : 545–554.
Horne R . Compliance, adherence, and concordance: implications for asthma treatment. Chest 2006; 130 (suppl): 65S–72S.
Reddel HK, Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009; 180 : 59–99.
Thomas M, Kay S, Pike J, Rosenzweig JR, Hillyer EV, Price D . The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J 2009; 18 : 41–49.
Hoskins G, Williams B, Jackson C, Norman P, Donnan P . Assessing asthma control in UK primary care: use of routinely collected prospective observational consultation data to determine appropriateness of a variety of control assessment models. BMC Fam Pract 2011; 12 : 105.
Pinnock H, Fletcher M, Holmes S, Keeley D, Leyshon J, Price D et al. Setting the standard for routine asthma consultations: a discussion of the aims, process and outcomes of reviewing people with asthma in primary care. Prim Care Respir J 2010; 19 : 75–83.
McKinstry B, Hammersley V, Burton C, Pinnock H, Elton RA, Dowell J et al. The quality, safety and content of telephone and face-to-face consultations: a comparative study. Qual Saf Health Care 2010; 19 : 298–303.
Gordon C, Galloway T . Review of Findings on Chronic Disease Self-Management Program (CDSMP) Outcomes: Physical, Emotional & Health-Related Quality of Life, Healthcare Utilization and Costs . Centers for Disease Control and Prevention and National Council on Aging: Atlanta, GA, USA, 2008.
Beasley R, Crane J . Reducing asthma mortality with the self-management plan system of care. Am J Respir Crit Care Med 2001; 163 : 3–4.
Ring N, Jepson R, Pinnock H, Wilson C, Hoskins G, Sheikh A et al. Encouraging the promotion and use of asthma action plans: a cross study synthesis of qualitative and quantitative evidence. Trials 2012; 13 : 21.
Jones A, Pill R, Adams S . Qualitative study of views of health professionals and patients on guided self-management plans for asthma. BMJ 2000; 321 : 1507–1510.
Bandura A . Self-efficacy: toward a unifying theory of behavioural change. Psychol Rev 1977; 84 : 191–215.
Gollwitzer PM, Sheeran P . Implementation intentions and goal achievement: a meta-analysis of effects and processes. Adv Exp Soc Psychol 2006; 38 : 69–119.
Google Scholar
Hardeman W, Johnston M, Johnston DW, Bonetti D, Wareham NJ, Kinmonth AL . Application of the theory of planned behaviour change interventions: a systematic review. Psychol Health 2002; 17 : 123–158.
Schwarzer R . Modeling health behavior change: how to predict and modify the adoption and maintenance of health behaviors. Appl Psychol 2008; 57 : 1–29.
Sniehotta F . Towards a theory of intentional behaviour change: plans, planning, and self-regulation. Br J Health Psychol 2009; 14 : 261–273.
Okelo SO, Butz AM, Sharma R, Diette GB, Pitts SI, King TM et al. Interventions to modify health care provider adherence to asthma guidelines: a systematic review. Pediatrics 2013; 132 : 517–534.
Grol R, Grimshaw RJ . From best evidence to best practice: effective implementation of change in patients care. Lancet 2003; 362 : 1225–1230.
Jusef L, Hsieh C-T, Abad L, Chaiyote W, Chin WS, Choi Y-J et al. Primary care challenges in treating paediatric asthma in the Asia-Pacific region. Prim Care Respir J 2013; 22 : 360–362.
Donabedian A . Evaluating the quality of medical care. Milbank Q 2005; 83 : 691–729.
Fardy HJ . Moving towards organized care of chronic disease. The 3+ visit plan. Aust Fam Physician 2001; 30 : 121–125.
CAS PubMed Google Scholar
Glasgow NJ, Ponsonby AL, Yates R, Beilby J, Dugdale P . Proactive asthma care in childhood: general practice based randomised controlled trial. BMJ 2003; 327 : 659.
Douglass JA, Goemann DP, Abramson MJ . Asthma 3+ visit plan: a qualitative evaluation. Intern Med J 2005; 35 : 457–462.
Beilby J, Holton C . Chronic disease management in Australia; evidence and policy mismatch, with asthma as an example. Chronic Illn 2005; 1 : 73–80.
The Department of Health. Asthma Cycle of Care. Accessed on 14 May 2014 at http://www.health.gov.au/internet/main/publishing.nsf/Content/asthma-cycle .
National Asthma Council Australia. Asthma and Respiratory Education Program. Accessed on 14 May 2014 at http://www.nationalasthma.org.au/health-professionals/education-training/asthma-respiratory-education-program .
Patel MR, Shah S, Cabana MD, Sawyer SM, Toelle B, Mellis C et al. Translation of an evidence-based asthma intervention: Physician Asthma Care Education (PACE) in the United States and Australia. Prim Care Respir J 2013; 22 : 29–34.
Armour C, Bosnic-Anticevich S, Brilliant M, Burton D, Emmerton L, Krass I et al. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax 2007; 62 : 496–502.
Roberts NJ, Mohamed Z, Wong PS, Johnson M, Loh LC, Partridge MR . The development and comprehensibility of a pictorial asthma action plan. Patient Educ Couns 2009; 74 : 12–18.
Henry RL, Gibson PG, Vimpani GV, Francis JL, Hazell J . Randomised controlled trial of a teacher-led asthma education program. Pediatr Pulmonol 2004; 38 : 434–442.
National Asthma Council Australia. Asthma Friendly Schools program. Accessed on 14 May 2014 at http://www.asthmaaustralia.org.au/Asthma-Friendly-Schools.aspx .
Download references
Author information
Authors and affiliations.
Asthma UK Centre for Applied Research, Centre for Population Health Sciences, The University of Edinburgh, Edinburgh, UK,
Hilary Pinnock & Elisabeth Ehrlich
NMAHP-RU, University of Stirling, Stirling, UK,
Gaylor Hoskins
Discipline of General Practice, University of Sydney, Sydney, NSW, Australia
Ron Tomlins
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Hilary Pinnock .
Ethics declarations
Competing interests.
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
Reprints and permissions
About this article
Cite this article.
Pinnock, H., Ehrlich, E., Hoskins, G. et al. A woman with asthma: a whole systems approach to supporting self-management. npj Prim Care Resp Med 24 , 14063 (2014). https://doi.org/10.1038/npjpcrm.2014.63
Download citation
Received : 23 June 2014
Revised : 15 July 2014
Accepted : 15 July 2014
Published : 16 October 2014
DOI : https://doi.org/10.1038/npjpcrm.2014.63
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Search Menu
- Sign in through your institution
- Advance Articles
- Supplements
- Author Guidelines
- Reviewer guidelines
- Submission Site
- Open Access
- Self-Archiving Policy
- Why Publish?
- Advertising & Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Paediatrics & Child Health
- About The Canadian Paediatric Society
- Editorial Board
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Case 1 diagnosis: allergy bullying, clinical pearls.
- < Previous
Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation
- Article contents
- Figures & tables
- Supplementary Data
Lopamudra Das, Michelle GK Ward, Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation, Paediatrics & Child Health , Volume 19, Issue 2, February 2014, Pages 69–70, https://doi.org/10.1093/pch/19.2.69
- Permissions Icon Permissions
A 12-year-old girl with a history of asthma presented to the emergency department with a three-day history of increased work of breathing, cough and wheezing. She reported no clear trigger for her respiratory symptoms, although she had noted some symptoms of a mild upper respiratory tract infection. With this episode, the patient had been using a short-acting bronchodilator more frequently than she had in the past, without the expected resolution of symptoms.
On the day of presentation, the patient awoke feeling ‘suffocated’ and her mother noted her lips to be blue. In the emergency department, her oxygen saturation was 85% and her respiratory rate was 40 breaths/min. She had significantly increased work of breathing and poor air entry bilaterally to both lung bases, with wheezing in the upper lung zones. She was treated with salbutamol/ipratropium and received intravenous steroids and magnesium sulfate. Her chest x-ray showed hyperinflation and no focal findings.
Her medical history revealed that she was followed by a respirologist for her asthma, had good medication adherence and had not experienced a significant exacerbation for six months. She also had a history of wheezing, dyspnea and pruritis with exposure to peanuts, chickpeas and lentils; she had been prescribed an injectible epinephrine device for this. However, her device had expired at the time of presentation. In the past, her wheezing episodes had been seasonal and related to exposure to grass and pollens; this presentation occurred during the winter. Further history revealed the probable cause of her presentation.
Although reluctant to disclose the information, our patient later revealed that she had been experiencing significant bullying at school, which was primarily related to her food allergies. Three days before her admission, classmates had smeared peanut butter on one of her schoolbooks. She developed pruritis immediately after opening the book and she started wheezing and coughing later that day. This event followed several months of being taunted with peanut products at school. The patient was experiencing low mood and reported new symptoms of anxiety related to school. The review of systems was otherwise negative, with no substance use.
The patient's asthma exacerbation resolved with conventional asthma treatment. Her pulmonary function tests were nonconcerning (forced expiratory volume in 1 s 94% and 99% of predicted) after her recovery. The trigger for her asthma exacerbation was likely multifactorial, related to exposure to the food allergen as well as the upper respiratory infection. A psychologist was consulted to assess the symptoms of anxiety and depression that had occurred as a result of the bullying. During the hospitalization, the medical team contacted the patient's school to provide education on allergy bullying, treatment of severe allergic reactions and its potential for life-threatening reactions with exposure to allergens. The medical team also recommended community resources for further education of students and staff about allergy bullying and its prevention.
Allergy bullying is a form of bullying with potentially severe medical outcomes. In recent years, it has gained increasing notoriety in schools and in the media. Population-based studies have shown that 20% to 35% of children with allergies experience bullying. In many cases (31% in one recent study [ 1 ]), this bullying is related directly to the food allergy. From a medical perspective, there are little published data regarding allergy bullying, and many health care providers may not be aware of the issue.
Allergy bullying can include teasing a child about their allergy, throwing food at a child, or even forcing them to touch or eat allergenic foods. Most episodes of allergy bullying occur at school, and can include episodes perpetrated by teachers and/or staff ( 2 ).
Allergy bullying can lead to allergic reactions, which may be mild or severe (eg, urticaria, wheezing, anaphylaxis), but may also lead to negative emotional consequences (sadness, depression) ( 2 ) and an overall decrease in quality of life measures ( 1 ). Adolescents commonly resist using medical devices, such as injectible epinephrine devices, and bullying may be a contributing factor for this ( 3 ). Attempting to conceal symptoms in a bullying situation may place children at risk for a worse outcome.
Physicians can play a key role in detecting allergy bullying and its health consequences. In many cases, children have not discussed this issue with their parents ( 1 ). Given the prevalence of bullying, its potential to lead to severe harm, including death, and the lack of awareness of this issue, clinicians should specifically ask about bullying in all children and teens with allergies. Physicians can also work with families and schools to support these children, educate their peers and school staff, and help prevent negative health outcomes from allergy bullying.
Online resources
www.anaphylaxis.ca − A national charity that aims to inform, support, educate and advocate for the needs of individuals and families living with anaphylaxis, and to support and participate in research. This website includes education modules for schools and links to local support groups throughout Canada.
www.whyriskit.ca/pages/en/live/bullying.php − A website for teenagers with food allergies; includes a segment that addresses food bullying.
www.foodallergy.org − Contains numerous resources for children and their families, including a significant discussion on bullying and ways to prevent it.
Allergy bullying is common but is often unrecognized as a factor in clinical presentations of allergic reactions.
Physicians should make a point of asking about bullying in patients with allergies and become familiar with resources for dealing with allergy bullying.
Physicians can play roles as advocates, educators and collaborators with the school system to help make the school environment safer for children with allergies who may be at risk for allergy bullying.
Google Scholar
| Month: | Total Views: |
|---|---|
| February 2017 | 2 |
| June 2017 | 2 |
| November 2017 | 3 |
| December 2017 | 6 |
| January 2018 | 3 |
| February 2018 | 5 |
| March 2018 | 7 |
| April 2018 | 10 |
| May 2018 | 8 |
| June 2018 | 13 |
| July 2018 | 3 |
| August 2018 | 5 |
| September 2018 | 8 |
| October 2018 | 6 |
| November 2018 | 8 |
| December 2018 | 22 |
| January 2019 | 1 |
| February 2019 | 8 |
| March 2019 | 3 |
| April 2019 | 13 |
| May 2019 | 9 |
| June 2019 | 5 |
| July 2019 | 17 |
| August 2019 | 12 |
| September 2019 | 15 |
| October 2019 | 6 |
| November 2019 | 3 |
| December 2019 | 4 |
| January 2020 | 8 |
| February 2020 | 10 |
| March 2020 | 16 |
| April 2020 | 44 |
| May 2020 | 65 |
| June 2020 | 102 |
| July 2020 | 119 |
| August 2020 | 104 |
| September 2020 | 221 |
| October 2020 | 286 |
| November 2020 | 391 |
| December 2020 | 336 |
| January 2021 | 456 |
| February 2021 | 492 |
| March 2021 | 661 |
| April 2021 | 646 |
| May 2021 | 662 |
| June 2021 | 507 |
| July 2021 | 389 |
| August 2021 | 484 |
| September 2021 | 628 |
| October 2021 | 860 |
| November 2021 | 752 |
| December 2021 | 627 |
| January 2022 | 599 |
| February 2022 | 698 |
| March 2022 | 922 |
| April 2022 | 913 |
| May 2022 | 728 |
| June 2022 | 450 |
| July 2022 | 299 |
| August 2022 | 406 |
| September 2022 | 668 |
| October 2022 | 956 |
| November 2022 | 915 |
| December 2022 | 771 |
| January 2023 | 649 |
| February 2023 | 707 |
| March 2023 | 769 |
| April 2023 | 646 |
| May 2023 | 748 |
| June 2023 | 428 |
| July 2023 | 356 |
| August 2023 | 387 |
| September 2023 | 567 |
| October 2023 | 743 |
| November 2023 | 691 |
| December 2023 | 524 |
| January 2024 | 493 |
| February 2024 | 667 |
| March 2024 | 743 |
| April 2024 | 767 |
| May 2024 | 750 |
| June 2024 | 333 |
| July 2024 | 283 |
| August 2024 | 464 |
Email alerts
Citing articles via, looking for your next opportunity.
- About Paediatrics & Child Health
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1918-1485
- Print ISSN 1205-7088
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Open access
- Published: 03 April 2020
Determinants of Acute Asthma Attack among adult asthmatic patients visiting hospitals of Tigray, Ethiopia, 2019: case control study
- Melaku Negash 1 ,
- Hagos Tsegabrhan 2 ,
- Teklit Meles 3 ,
- Degena Bahrey Tadesse 1 ,
- Gebreamlak Gidey 4 ,
- Yemane Berhane 5 ,
- Kibrom Berhanu 6 &
- Tsgalem Haylemaryam 7
Asthma Research and Practice volume 6 , Article number: 1 ( 2020 ) Cite this article
5143 Accesses
3 Citations
3 Altmetric
Metrics details
Introduction
Acute asthma attack is one of the most common causes of visits to hospital emergency departments in all age groups of the population and accounts for the greater part of healthcare burden from the disease. Despite, Acute asthma attack is an important public health problem that affects not only the patients, but also to the family, health professionals, health care institutions and development of the nation, little is known about the risk factors of acute asthma attack.
Therefore, this study is aimed to investigate the determinants of acute asthma attack among.
The aim of this study was to assess the determinant factors of acute asthma attack among adult asthmatic patients visiting general hospitals of central zone, Tigray, Ethiopia, 2019.
Hospital based unmatched case control study design was conducted in general hospitals of central zone of Tigray, Ethiopia 2019. Data were collected using pretested interviewer administered questionnaire. A total of 289 study subjects (96 cases &193 controls) were selected by systematic random sampling. Data were entered to Epi data version 3.1 then exported to SPSS version 23 for analysis. Bivariate logistic regression was employed to examine the statistical association between dependent and independent variables. Variables with p value < 0.25 in binary logistic regression were entered to multivariable logistic regression model and variables with p value < 0.05 was taken as significant determinants of the outcome variable.
A total of 96 adult asthmatic patients who have acute asthma attack (cases) and 193 adult asthmatic patients without attack (controls)) with 100% response rate were participated in this study. Upper Respiratory tract Infection [AOR = 6.835,95% CI = 3.285,14.222], Season [AOR =2.204,95% CI = 1.011,4.805] kitchen smoke [AOR = 2.307,95%CI1.010,5.272]& sleep apnea [AOR = 9.254, 5%CI =3.563,25.460] were significantly associated with acute asthma exacerbation.
Asthma is a long-term inflammatory disease of the respiratory system which is characterized by wheezing, shortness of breath, chest tightness. Globally it affects approximately 300 million people and is estimated to rise to 400 million by 2025 globally [ 1 , 2 ]. And it is ranked 16th among the leading causes of disability and 28th among the leading causes of burden of disease, as measured by disability adjusted life years (DALYs) [ 3 ].
According to Croatian medical journal 2013, an estimate of asthma prevalence in Africa, was 49.7 million in the age of < 15 years (13.9%), < 45 years 102.9 million (13.8%), and in total population 119.3 million (12.8%) in 2010 [ 4 ].
Asthma exacerbation is defined as a worsening of shortness of breath, cough, wheezing, or chest tightness. If not treated immediately there will be increase in flow resistance causing increased work of breathing, gas exchange inefficiency, respiratory muscle tiredness and finally hypercapnic and hypoxemic respiratory failure [ 5 ]. This implies that acute asthma attack is a significant public health problem that affects patients with their parents or families and the community through labor and school loss, frequent emergency clinic visits, a poor quality of life hospitalizations and finally death [ 6 ]. According to Centers for Disease Control and prevention (CDC) report, More than 11 million people reported having an acute asthma attack [ 7 ].
Despite, in Ethiopia little is known about how risk factors are associated with exacerbation, according to asthma severity and the relative importance of the risk factors. This may be the reason for no policy and strategy to ascertain and acting out of effective intervention in order to reduce the burden of acute asthma attack [ 8 ]. Therefore, this study is aimed to full fill this gap.
Study setting and study design
Hospital based unmatched case control study was conducted in the selected general Hospitals of Central zone of Tigray from November 2018 to July 2019.
Study population and sample size determination
Source population.
All adult asthmatic patients visited to emergency unit who have acute asthma attack.
All adult patients diagnosed as asthma but without acute asthmatic attack who visited the OPD and the regular follow-up unit during the data collection period.
Study population
All selected adult asthmatic patients visited to emergency unit who have acute asthma attack during the data collection period.
All selected adult patients diagnosed as asthma but without acute asthmatic attack who visited the OPD and the regular follow-up unit during the data collection period.
Eligibility criteria
Inclusion criteria.
Adult asthmatic patients who have acute asthma attack during the data collection period.
Adult asthmatic patient without acute asthma attack during the data collection period.
Exclusion criteria
Patients with any history of pulmonary embolism, chronic obstructive pulmonary disease, active pulmonary TB, known congestive heart failure and known mechanical obstruction.
Sample size determination
Sample size was calculated from Previous study conducted in Uganda [ 9 ],using Epi info version 7. sample size was determined based on the assumption of confidence level = 95%; Power = 80%; Odds ratio = 2.132 with case to control ratio = 1:2, proportion of among controls 37.2%, proportion of among cases = 55.8%.
Therefore, the required sample size for cases was =92 where as for the controls =183 and the overall sample size was = 275 then after adding 5% non-response rate, the total sample size was 289. Finally, a sample size for cases was 96 and for controls 193.
Sampling technique and procedure
The total sample size was allocated to each hospital proportionally based on the number of patients who attend in the selected hospitals. A total number of 585(case 165, control.420) patients attended at the selected Hospitals with in 2 months of the previous year (April 1 to May 30–2018). Systematic random sampling method was applied in each hospital to select 289 participants.
Study Variables
Dependent variable.
Acute asthma attack.
Independent variables
Socio-demographic variables.
Age, Gender, Marital status, Residence, Educational level, Employment status and Occupational status.
Behavioral factors
Exercise, vigorous activity Smoking cigarette.
Environmental factors
Humidity, Kitchen smoke, dust, Season.
Medical and Clinical characteristics
URTI, Sleep apnea, Missing follow-up / appointments,
Operational definitions
Those who present with cough, wheezing and difficulty of breathing and diagnosed asthma by physician [ 10 ].
Acute Asthma Attack
Those who present with worsening of wheezing, shortness of breath, cough, chest tightness and diagnosed as acute asthma attack by physician [ 10 ].
Smoker:( daily smoker and non-daily smoker) those who currently smokes or those who quit smoking less than 1 year before the assessment [ 10 ].
Passive smoker: Smoke inhaled involuntarily by non-smokers [ 11 ].
Nonsmoker: Respondents who report never smoke those who quit smoking greater than 1 year before the assessment.
Vigorous activity: participants doing activity more than 10 min continuously, that increases breathing, like carrying or lifting heavy loads, digging or construction work, cutting fire wood [ 11 ].
Data collection tool
Structured questionnaire was used to collect the data which was adapted from different literatures [ 9 , 12 , 13 , 14 ]. The questionnaire contains four parts: socio-demographic, environmental factors, behavioral factors, and Medical &Clinical characteristics.
Data collection procedures
Data were collected from cases and controls using structured questionnaire and checklists through face-to-face interview and from patients chart review respectively.
Twelve BSc nurses as data collectors and three senior nurse supervisors were recruited for the data collection, Then data from cases were collected after they take all the necessary medical care and they recover from their attack whereas from the controls data were collected after they have completed their assessment by physician and at the last record reviews from their chart. Participants were identified as having upper respiratory tract infection and Obstructive sleep apnea from their medical charts which was diagnosed by senior physicians. This is to mean that, it was just suspected clinically by the time of the acute event. The reason we obeyed to use clinically diagnosis for obstructive sleep apnea is that, there is no accesses of modern diagnostic modality like polysomnography in the study area which was Tigray regional state not only in the study area but also in the country Ethiopia as a whole. The evaluation protocol that we use were a single evaluation visit for each case and even those who have follow-up and developed acute asthma attack were included .
Data quality control techniques
Data quality was ensured by training of data collectors and supervisors before data collection period. 5% of the questionnaire was pre-tested in Shire Hospital which was not included in the actual data collection. Based on the findings of the pre-test, questionnaire was modified. The filled questionnaire was checked for completeness and accuracy by data collectors, supervisors and principal investigator each day.. The questionnaire was translated into Tigrigna language for better understanding to both the data collectors and respondents and then back translated into English by another expert to ensure accuracy and consistency.
Data analysis procedures
Data were entered in to Epi data version 3.1 and analyzed using SPSS version 23.0. The degree of association between independent and dependent variables were assessed using adjusted odds ratio with 95% confidence interval. Variables < 0.25 p -value in binary logistic regression were entered to multivariable logistic regression model to control the potential confounding variables. Variables with p-value less than 0.05 in multivariable logistic regression model were taken as significantly associated factors. Variance inflation factor (VIF) was used to assess Multicollinearity between the independent variables. Hosmer and Lemeshow goodness fit model were used to check model fitness.
Ethical consideration
Ethical clearance was obtained from Mekelle University College of health sciences institutional review board (IRB). A subsequent permission was also obtained from Tigray teaching hospitals. Respondents were informed about the purpose of the study and the interview was conducted after receiving the written consent from participants. Confidentiality of the data/information was secured and was not used for other purposes.
Sociodemographic characteristic of study participants
Among the participants, 67.7% (65) of the cases and 60.6% (117) of the controls were females. The median ages of participants were 43 years with interquartile range (IQR) of 26.5 years among cases and 43 median ages with interquartile range (IQR) of 22 for control.
The educational status, one third 33.3% (32) of the cases and 24.9% (48) of the controls were collage and above, where as 14.6% (14) of the cases and 16.6% (32) of the controls were unable to read and write. The majority of the cases 63.5% (61) and 60.1% (116) of the controls were married (Table 1 ).
Behavioral characteristics of study participants
Among the participants, 2.1% (2) of the cases and 1.1% (6) of the controls were smokers.in parallel with this 3.1% of the cases and 4.7% of the control were passive smokers. Regarding vigorous activity 37.5% (36) of the cases and 23.8% (46) of the controls were do vigorous activity. Majority of the participants 72.9% (70) of the cases and 58% (112) of the controls were doing exercise.
Medical & clinical characteristics of study participants
Among the participants, 44.8% (43) of the cases and 13.5% (26) of the controls had Upper Respiratory Tract Infections (URTI) and 29.2% (28) of the cases and few of the controls 5.2% (10) had obstructive sleep apnea.
Among the participants, 31.3% (30) of the cases and 20.7% (40) of the controls had Missing follow up.
Environmental characteristics of study participants
Regarding the seasons of a year, spring season (April, May, June) were the season with high percentage 37.7% (109) of acute asthma attack than the autumn season. Majority of the participants 79.5% (230) were open their window/door while they were cooking. Concerning the kitchen of the participants 32.3% (31) of the cases and 20.2% (39) of the control’s kitchen have no kitchen smoke (chimney) (Table 2 ).
Unmatched case control study with 96 cases and 193 controls was conducted to show the determinants of acute asthma attack among adult asthmatic patients visiting general hospitals of central zone, Tigray, Ethiopia.
Having URTI increases the occurrence of acute asthma attack 6.8 times [AOR = 6.835,95% CI = 3.285,14.222] than those who have not upper respiratory tract infection (URTI) (Table 3 ).
This is consistent with the studies conducted in Gondar, Uganda and Ireland [ 9 , 12 , 15 ].
The association might be due to the mechanism of airway inflammation,mucus hyper secretion, and bronchial hyper responsiveness [ 16 ]. In contrast to this study upper respiratory tract infections was no risk factor for acute asthma exacerbation on the study conduct in Pretoria and New Zealand [ 14 , 17 ]. This difference might be due to difference in health care seeking behavior of the participants in this study.
This study revealed that, sleep apnea was strongly associated with the occurrence of acute asthma exacerbation. Those who have sleep apnea are 9.5 times more likely to run in to acute asthma exacerbation than those who have not sleep apnea [AOR = 9.524, 95% CI = 3.563, 25.460].
This findings is comparable with a study done in Gondar and USA [ 12 , 18 ].
The possible reason is the fact that sleep apnea lead to the worsening of asthma control in patients with concomitant sleep apnea secondary to bronchoconstriction as a result of increase vagal tone while sleeping [ 19 ].
The result of this study shows that the odds of having acute asthma in Spring season was 2.2 times higher than the odds of having acute asthma attack in the autumn season [AOR = 2.204,95% CI = 1.011,4.805]. This is consistent with a study conducted in Canada in which spring season was triggering factor for asthma exacerbation [ 20 ]. Seasonal variation is the risk factors for acute asthma attack especially pollens appearing seasons like spring season exacerbates acute asthma attack. This may be due to the reason that during the spring, tree pollen, mold spores and grass have the power to inflame and narrow the air passages of people who have asthma [ 21 ].
The result of this study was different from a study conducted in Spain which was resulting winter season as higher risk of developing acute asthma attack [ 22 ]. The difference could be arisen from seasonal variation between the study areas, due to the influence of temperature and humidity.
In this study, Kitchen smoke (chimney) is highly associated with risk of acute asthma exacerbation.
Those who have no kitchen smoke in their kitchen were 2.3 times at risk to develop acute asthma exacerbation [AOR = 2.307,95%CI = 1.010,5.2725] than those who have kitchen smoke. This finding is comparable with the study conducted in India [ 13 ]. This is due to the fact that kitchen smoke (chimney) is a way that helps in removing the smokes and fumes from the kitchen and making it clean and smoke free which result in reduction of indoor air pollution and prevents acute asthma exacerbation [ 23 ]. Inhaling harmful smoke can inflame lungs and airway, causing them to swell and block oxygen. This can lead to acute asthma exacerbation [ 24 ]
In this study the determinant factors of acute asthma attack were spring season, presence of upper respiratory tract infection (URTI), having no Kitchen smoke in their kitchen and having obstructive sleep apnea.
Limitations
The diagnosis of respiratory tract infections and sleep apnea was empirical (without laboratory) and all measures used were based on self-reporting, this might end up with social desirability bias. This study may have recall bias, since some of the information was based on the recall of the study participants. Unavailability of studies on acute asthma exacerbation.
Availability of data and materials
The datasets used and analyzed during the current study are presented within the manuscript and available from the corresponding author on reasonable request.

Abbreviations
Adjusted Odds Ratio
Confidence Interval
Crude Odds Ratio
Central Statistical Agency
Interquartile Range
National Health Interview Survey
Out Patient Department
Tigray Region Health Development Agency
Upper Respiratory Tract Infection
Variance Inflation Factor
Adams, JY., Sutter, M.E. & Albertson, T.E. The Patient with Asthma in the Emergency Department. Clinic Rev Alleg Immunol 43, 14-29 (2012). https://doi.org/10.1007/s12016-011-8273-z .
Shah R , Saltoun CA . Chapter 14: Acute severe asthma (status asthmaticus). Allergy and Asthma Proceedings, 2012; 33(Supplement 1):S47-S50. Acute severe asthma. InAllergy and Asthma proceedings 2012 (Vol. 33, No. 3, p. 47). OceanSide Publications..
The Global Asthma Report 2018. Auckland, New Zealand: Global Asthma Network, 2018.
Adeloye D, Chan KY, Rudan I, Campbell H. An estimate of asthma prevalence in Africa: a systematic analysis. Croat Med J. 2013;54(6):519–31.
Article Google Scholar
Park HW, Tantisira KG. Genetic signatures of asthma exacerbation. Allergy, Asthma Immunol Res. 2017;9(3):191–9.
Article CAS Google Scholar
Stewart WF, Ricci JA, Chee E, Morganstein D. Lost productive work time costs from health conditions in the United States: results from the American Productivity Audit. J Occup Environ Med. 2003;45(12):1234–46.
CDC , National Health Interview Survey (NHIS) 2014.
Google Scholar
Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: Origin, effect and prevention. J Allergy Clin Immunol. 2011;128:1165–74.
Sanya RE, Kirenga BJ, Worodria W, Okot-Nwang M. Risk factors for asthma exacerbation in patients presenting to an emergency unit of a national referral hospital in Kampala, Uganda. Afr Health Sci. 2014;14(3):707–15.
Riley L, Gouda H, Cowan M. Noncommunicable Diseases Progress Monitor, 2017: World Health Organization; 2017.
Ethiopia steps report on risk factors for Chronic Non Communicable Diseases and prevalence of selected NCDs. Ethiopia public Health institute. 2016 . .
Belachew SA, Erku DA, Yimenu DK, Gebresillassie BM. Assessment of predictors for acute asthma attack in asthmatic patients visiting an Ethiopian hospital: are the potential factors still a threat? Asthma Res Pract. 2018;4(1):8.
Sharma GL, Choudhary GS. Assessment of Risk Factors for Acute Asthma Attack in Asthmatic Patients: A Hospital Based Study. Int Arch BioMed Clin Res. 2018;4(4):46–8.
Geyser M, Rheeder P. Risk factors precipitating exacerbations in adult asthma patients presenting at Kalafong Hospital, Pretoria. S Afr Fam Pract. 2008;50(4):67–e.
Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 2003;307(6910):982–6.
Fraenkel DJ, Bardin PG, Sanderson G, et al. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 2009;151(3):879–86.
Kolbe J, Fergusson W, Vamos M, Garrett J. Case-control study of severe life-threatening asthma (SLTA) in adults. Thorax. 2002;57(4):317–22.
De-Lei K, Zheng Q, Hui S, Hong Y. Association of Obstructive Sleep Apnea with Asthma exacerbation; 2017.
Alkhalil M, Schulman E, Getsy J. Obstructive sleep apnea syndrome and asthma: what are the links? J Clin Sleep Med. 2009;5(01):71–8.
Tarlo S, Broder I, Corey P, et al. A case-control study of the role of cold symptoms and other historical triggering factors in asthma exacerbations. Can Respir J. 2000;7(1):42–8.
Surrena H, editor. Handbook for Brunner and Suddarth’s textbook of medical-surgical nursing. Lippincott Williams & Wilkins; 2010.
Pola-Bibian B, et al. Asthma exacerbations in a tertiary hospital: clinical features, triggers, and risk factors for hospitalization. J Investig Allergol Clin Immunol. 2016: 0 . https://doi.org/10.18176/jiaci.0128 .
Eisner M, et al. Exposure to indoor combustion and adult asthma outcomes: environmental tobacco smoke, gas stoves, and woodsmoke. Thorax. 2002;57(11):973–8.
Smeltzer SC, Bare BG, Hinkele JL, Cheever KH. Brunner and Suddath’s Text Book of Medical Surgical 2010. Wolters Kluwer Health:Lippincott Williams & Wilkins. Nursing, vol. 1. 12th ed. p. 622.
Download references
Acknowledgments
Authors thanks to public general hospitals of central zone Tigray, Ethiopia for their co-operation, to data collectors, supervisors, for the health staffs of the hospitals and to the study participants for their valuable information.
Not applicable.
Author information
Authors and affiliations.
Department of adult health nursing ,school of Nursing, Aksum University, Aksum, Ethiopia
Melaku Negash & Degena Bahrey Tadesse
Department of Psychiatric, Mekelle University, Mekelle, Ethiopia
Hagos Tsegabrhan
Adwa General Hospital, Adwa, Ethiopia
Teklit Meles
Department of midwifery, Aksum University, Aksum, Ethiopia
Gebreamlak Gidey
college of medicine and health science, Adigrat university, Adigrat, Ethiopia
Yemane Berhane
Maternity and reproductive health nursing, Mekelle University, Mekelle, Ethiopia
Kibrom Berhanu
Department of Emergency and critical care nursing, Mekelle University, Mekelle, Ethiopia
Tsgalem Haylemaryam
You can also search for this author in PubMed Google Scholar
Contributions
MN: was made substantially contributions to conceived and designed the study, analysis the data, methodology, data interpretation and wrote the final manuscript.TM, DB, GG,YB, had equally contributed to analysis and interpretation of the data. Whereas HT, TH and KB substantial contribution in reviewing overall the study in analysis, interpretation of data, have drafted the manuscript and substantively revised the work. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Melaku Negash .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance was obtained from Mekelle University College of health sciences institutional review board (IRB). Official supportive letters were obtained from Regional Health Bureau (TRHB) and central zone health office. Respondents were informed about the purpose of the study and the interview was conducted after receiving the written consent from participants. The right of participants to withdraw from the study at any time, without any precondition were secured and participants were informed. Confidentiality of the data/information was secured and was not used for other purposes. No personal identifiers was used on the questionnaire. To maintain confidentiality, data collector was recruited from the study unit.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
Annex I: English version structured interview questionnaire.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Negash, M., Tsegabrhan, H., Meles, T. et al. Determinants of Acute Asthma Attack among adult asthmatic patients visiting hospitals of Tigray, Ethiopia, 2019: case control study. asthma res and pract 6 , 1 (2020). https://doi.org/10.1186/s40733-020-00054-w
Download citation
Received : 07 December 2019
Accepted : 17 March 2020
Published : 03 April 2020
DOI : https://doi.org/10.1186/s40733-020-00054-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute asthma attack
- Determinants
Asthma Research and Practice
ISSN: 2054-7064
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Assessment and...
Assessment and management of adults with asthma during the covid-19 pandemic
Read our latest coverage of the coronavirus pandemic.
- Related content
- Peer review
- Thomas Beaney , academic clinical fellow in primary care 1 ,
- David Salman , academic clinical fellow in primary care 1 ,
- Tahseen Samee , specialist registrar in emergency medicine 2 ,
- Vincent Mak , consultant in respiratory community integrated care 3
- 1 Department of Primary Care and Public Health, Imperial College London, London, UK
- 2 Barts Health NHS Trust, London, UK
- 3 Imperial College Healthcare NHS Trust, London, UK
- Correspondence to: T Beaney Thomas.beaney{at}imperial.ac.uk
What you need to know
In patients with pre-existing asthma, a thorough history and structured review can help distinguish an asthma exacerbation from covid-19 and guide management
In those with symptoms of acute asthma, corticosteroids can and should be used if indicated and not withheld on the basis of suspected covid-19 as a trigger
Assessment can be carried out remotely, ideally via video, but have a low threshold for face-to-face assessment, according to local arrangements
A 35 year old man contacts his general practice reporting a dry cough and increased shortness of breath for the past three days. He has a history of asthma, for which he uses an inhaled corticosteroid twice daily and is now using his salbutamol four times a day. Because of the covid-19 outbreak, he is booked in for a telephone consultation with a general practitioner that morning.
Asthma is a condition commonly encountered in primary care, with over five million people in the UK prescribed active treatment. 1 While seemingly a routine part of general practice, asthma assessment is a particular challenge in the context of the covid-19 pandemic, given the overlap in respiratory symptoms between the two conditions and the need to minimise face-to-face assessment. Over 1400 people died from asthma in 2018 in England and Wales, 2 while analyses of non-covid-19 deaths during the covid-19 outbreak have shown an increase in deaths due to asthma, 31 highlighting the need to distinguish the symptoms of acute asthma from those of covid-19 and manage them accordingly.
This article outlines how to assess and manage adults with exacerbations of asthma in the context of the covid-19 outbreak ( box 1 ). We focus on the features differentiating acute asthma from covid-19, the challenges of remote assessment, and the importance of corticosteroids in patients with an asthma exacerbation.
Asthma and covid-19: what does the evidence tell us?
Are patients with asthma at higher risk from covid-19.
Some studies, mostly from China, found lower than expected numbers of patients with asthma admitted to hospital, suggesting they are not at increased risk of developing severe covid-19. 3 4 5 However, these reports should be viewed cautiously, as confounding by demographic, behavioural, or lifestyle factors may explain the lower than expected numbers. Recent pre-print data from the UK suggest that patients with asthma, and particularly severe asthma, are at higher risk of in-hospital mortality from covid-19. 6 In the absence of more conclusive evidence to indicate otherwise, those with asthma, particularly severe asthma, should be regarded as at higher risk of developing complications from covid-19. 7
Can SARS-CoV-2 virus cause asthma exacerbations?
Some mild seasonal coronaviruses are associated with exacerbations of asthma, but the coronaviruses causing the SARS and MERS outbreaks were not found to be. 8 9 In the case of SARS-CoV-2 virus, causing covid-19, data from hospitalised patients in China did not report symptoms of bronchospasm such as wheeze, but the number of patients with pre-existing asthma was not reported. 10 More recent pre-print data from hospitalised patients in the UK identified wheeze in a minority of patients with Covid-19. 11 Given the overlap of symptoms, such as cough and shortness of breath, until further published data emerges, SARS-CoV-2 may be considered as a possible viral trigger in patients with an asthma attack.
What you should cover
Challenges of remote consultations.
Primary care services have moved towards telephone triage and remote care wherever possible to minimise the risk of covid-19 transmission. This brings challenges to assessment as visual cues are missing, and, unless the patient has their own equipment, tests involving objective measurement, such as oxygen saturation and peak expiratory flow, are not possible. In mild cases, assessment via telephone may be adequate, but, whenever possible, we recommend augmenting the consultation with video for additional visual cues and examination. 12 However, many patients, particularly the elderly, may not have a phone with video capability. If you are relying on telephone consultation alone, a lower threshold may be needed for face-to-face assessment.
Presenting symptoms
Start by asking the patient to describe their symptoms in their own words. Note whether they sound breathless or struggle to complete sentences and, if so, determine whether immediate action is required. If not, explore what has changed, and why the patient has called now. The three questions recommended by the Royal College of Physicians—asking about impact on sleep, daytime symptoms, and impact on activity—are a useful screening tool for uncontrolled asthma. 13 Alternative validated scores, such as the Asthma Control Questionnaire and Asthma Control Test, which include reliever use, are also recommended. 14 In assessing breathlessness, the NHS 111 symptom checker contains three questions—the answers may arise organically from the consultation, but are a useful aide memoire:
Are you so breathless that you are unable to speak more than a few words?
Are you breathing harder or faster than usual when doing nothing at all?
Are you so ill that you’ve stopped doing all of your usual daily activities?
Consider whether an exacerbation of asthma or covid-19 is more likely. Both can present with cough and breathlessness, but specific features may indicate one over the other (see box 2 ). Do the patient’s current symptoms feel like an asthma attack they have had before? Do symptoms improve with their reliever inhaler? Do they also have symptoms of allergic rhinitis? Pollen may be a trigger for some people with asthma during hay fever season.
History and examination features helping distinguish asthma exacerbation from covid-19 10 11 14 15 16
Exacerbation of asthma*.
Improvement in symptoms with reliever inhaler
Diurnal variation
Absence of fever
Coexisting hay fever symptoms
Examination:
Reduced peak expiratory flow
Close contact of known or suspected case
Dry continuous cough
Onset of dyspnoea 4-8 days into illness
Flu-like symptoms including fatigue, myalgia, headache
Symptoms not relieved by inhaler
Absence of wheeze
Peak expiratory flow may be normal
*Note SARS-CoV-2 infection may be a trigger for an asthma exacerbation
Risk factors and medications
To assess the risk of deterioration, ask specifically about any previous hospital admissions for asthma and about oral corticosteroid use over the past 12 months. Does the patient have any other high risk conditions or are they taking immunosuppressive drugs? Ask the patient if they smoke and take the opportunity to offer support to quit.
Are they prescribed an inhaled corticosteroid (ICS) or a long acting β agonist (LABA) and ICS combination inhaler? Are they using this regularly? Are they using a spacer device, and do they have a personal asthma action plan to guide management?
Psychosocial factors
Taking a psychosocial history can be more challenging over the telephone, where cues are harder to spot. Lessons from asthma deaths have shown that adverse psychosocial factors are strongly associated with mortality. 14 17 These include a history of mental health problems, lack of engagement with healthcare services, and alcohol or drug misuse, along with employment and income problems. Social isolation is also a risk factor, which may be exacerbated during social distancing measures. 17 The covid-19 outbreak is an anxious time for many patients, and symptoms of anxiety can contribute to the overall presentation.
Examination
In remote assessment, video can help guide decision making, and we recommend its use in asthmatic patients presenting with acute symptoms. First, assess the general appearance of the patient. A fatigued patient sitting up in bed, visibly breathless, and anchoring their chest will raise immediate concerns, as opposed to someone who is walking around while talking. Vocal tone and behaviour may indicate any contributing anxiety. Observe if the patient can speak in complete sentences, listen for audible wheeze, and count the respiratory rate. Assess the work of breathing, including the use of accessory muscles, and consider the use of a chaperone where appropriate. The Roth score is not advocated for assessment of covid-19 or asthma. 18
Further objective assessment can be made, such as measuring peak expiratory flow (PEF). If the patient does not have a PEF device at home, one can be prescribed, though this may not be feasible in an acute scenario. We recommend that PEF technique be witnessed via video to assess reliability. Silent hypoxia may be a feature of covid-19, and oxygen saturations should be measured if this is a concern. 19 In some regions, oxygen saturation probe delivery services are being implemented, which may facilitate this. Heart rate can also be provided by the patient if they use conventional “wearable” technology, although, given the potential inaccuracies with different devices, the results should not be relied on. 20 If time allows, inhaler technique can also be checked.
What you should do
Determine the most likely diagnosis.
Decide on the most likely diagnosis on the basis of the history and clinical features (see box 2 and fig 1 ) or consider whether an alternative or coexisting diagnosis is likely, such as a bacterial pneumonia or pulmonary embolus. If you suspect covid-19 without asthmatic features, manage the patient as per local covid-19 guidance.

Assessment and management of patients with known asthma during the covid-19 outbreak 14
- Download figure
- Open in new tab
- Download powerpoint
Determine severity and decide if face-to-face assessment is necessary
If asthmatic features are predominant, determine severity and treat according to Scottish Intercollegiate Guidelines Network (SIGN) and British Thoracic Society (BTS) guidance ( fig 1 ). 14 If the patient cannot complete sentences or has a respiratory rate ≥25 breaths/min, treat the case as severe or life threatening asthma and organise emergency admission. A peak expiratory flow (PEF) <50% of best or predicted or a heart rate ≥110 beats/min also indicate severe or life threatening asthma. If the patient does not meet these criteria, treat as a moderate asthma attack—a PEF of 50-75% of best or predicted helps confirm this. If they do not have a PEF meter, or if you are unsure as to severity, brief face-to-face assessment to auscultate for wheeze and assess oxygen saturations can help confirm the degree of severity and determine if the patient may be suitable for treatment at home with follow-up. Do not rely solely on objective tests and use clinical judgment to decide on the need for face-to-face assessment, based on knowledge of the patient, risk factors, and any adverse psychosocial circumstances.
Wheeze has been reported as a presenting symptom in a minority of patients with confirmed covid-19, and it may be difficult to rule out the presence of SARS-CoV-2 via remote assessment. 11 We recommend that, when a face-to-face assessment is needed, it should take place via local pathways in place to safely assess patients with suspected or possible covid-19—for example, at a local “hot” clinic. At present, performing a peak flow test is not considered to be an aerosol generating procedure, but the cough it may produce could be, so individual risk assessment is advised. 21 Consider performing PEF in an open space or remotely in another room via video link. Any PEF meter should be single-patient use only and can be given to the patient for future use.
Initial management when face-to-face assessment is not required
For moderate asthma exacerbations, advise up to 10 puffs of a short acting β agonist (SABA) inhaler via a spacer, administered one puff at a time. There is no evidence that nebulisers are more effective: 4-6 puffs of salbutamol via a spacer is as effective as 2.5 mg via a nebuliser. 22 Alternatively, if the patient takes a combined inhaled corticosteroid and long acting β agonist (LABA) preparation, then maintenance and reliever therapy (MART) can be used according to their action plan. 14 Management of an acute exacerbation should not rely solely on SABA monotherapy, so advise patients to follow their personal asthma action plan and continue corticosteroid treatment (or start it if they were not taking it previously) unless advised otherwise ( box 3 ). Antibiotics are not routinely recommended in asthma exacerbations.
Risks and benefits of inhaled and oral corticosteroids in asthma and covid-19
There is substantial evidence for the benefits of steroids in asthma. Regular use of inhaled steroids reduces severe exacerbations of asthma 23 and the need for bronchodilators, 24 while the prompt use of systemic corticosteroids during an exacerbation reduces the need for hospital admissions, use of β agonists, 25 and relapses. 26
The evidence for corticosteroid use in early covid-19 is still emerging. A systematic review of steroid use in SARS reported on 29 studies, 25 of which were inconclusive and four of which suggested possible harm (diabetes, osteoporosis, and avascular necrosis) but no reported effects on mortality. 27 WHO have cautioned against the use of systemic corticosteroids for the treatment of covid-19 unless indicated for other diseases. 28
In light of the strong evidence of benefits in patients with asthma, inhaled and oral corticosteroids should be prescribed if indicated in patients with symptoms of bronchoconstriction. Steroids should not be withheld on the theoretical risk of covid-19 infection, in line with guidance from the Primary Care Respiratory Society (PCRS), British Thoracic Society (BTS), and Global Initiative for Asthma (GINA). 15 22 29
Response to initial SABA or MART treatment can be assessed with a follow-up call at 20 minutes. If there is no improvement, further treatment may be necessary at a local hot clinic for reviewing possible covid-19, emergency department, or direct admission to an acute medical or respiratory unit depending on local pathways. For those who do respond, BTS-SIGN and GINA advise starting oral corticosteroids in patients presenting with an acute asthma exacerbation (such as prednisolone 40-50 mg for 5-7 days). 14 15 There is an increasing move in personalised asthma action plans to early quadrupling of the inhaled corticosteroid dose in patients with deteriorating control for up to 14 days to reduce the risk of severe exacerbations and the need for oral steroids. 15 30 However, there may be a ceiling effect on those who are already on a high dose of inhaled corticosteroid (see BTS table 14 ), so quadrupling the dose may not be effective in this group of patients. A personalised asthma action plan is an extremely helpful guide to treatment and should be completed or updated for all patients.
Follow-up and safety-netting
We recommend that all patients with moderate symptoms are followed up via remote assessment within 24 hours. Asthma attacks requiring hospital admission tend to develop relatively slowly over 6-48 hours. 14 However, deterioration can be more rapid, and symptoms can worsen overnight. Patients should be advised to look out for any worsening breathing or wheeze, lack of response to their inhalers, or worsening PEF. They should receive clear advice on what to do, including use of their reliever, and who to contact (such as the local out-of-hours GP provider, 111, or 999). With potential long waits for remote assessment, particularly out of hours, they should be advised to have a low threshold to call 999 if their symptoms deteriorate. If covid-19 infection is also suspected, advise them to isolate for seven days from onset of symptoms and arrange testing, according to the latest guidance. 7
How this article was created
We performed a literature search using Ovid, Medline, and Global Health databases using the search terms (asthma OR lung disease OR respiratory disease) AND (coronavirus OR covid-19)). Articles from 2019-20 were screened. We also searched for specific guidelines, including NICE, British Thoracic Society, Scottish Intercollegiate Guidelines Network, Primary Care Respiratory Society, European Respiratory Society, International Primary Care Respiratory Group, Global Initiative for Asthma, and the American Academy of Allergy, Asthma and Immunology.
Education into practice
Do you feel confident in completing personalised asthma plans in collaboration with patients?
How often do you start or increase inhaled corticosteroids in patients at initial presentation with an exacerbation of asthma?
If you manage a patient with acute asthma remotely, what safety netting advice would you give and how could you check understanding?
How patients were involved in the creation of this article
No patients were involved in the creation of this article.
This is part of a series of occasional articles on common problems in primary care. The BMJ welcomes contributions from GPs.
Contributors: TB and TS conceived the article. TB, DS, and TS carried out the literature review and wrote the initial drafts. All four authors contributed to editing and revision, and VM provided expert advice as a respiratory specialist. All authors are guarantors of the work.
Competing interests: We have read and understood BMJ policy on declaration of interests and have no relevant interests to declare.
Provenance and peer review: Commissioned, based on an idea from the author; externally peer reviewed.
- Mukherjee M ,
- Stoddart A ,
- ↵ Asthma UK. Asthma facts and statistics. https://www.asthma.org.uk/about/media/facts-and-statistics/ .
- Scabini S ,
- Mornese Pinna S ,
- Di Perri G ,
- De Rosa FG ,
- Williamson E. ,
- Walker AJ ,
- Bhaskaran KJ ,
- ↵ Public Health England. Guidance on social distancing for everyone in the UK [Withdrawn]. 2020. https://www.gov.uk/government/publications/covid-19-guidance-on-social-distancing-and-for-vulnerable-people/guidance-on-social-distancing-for-everyone-in-the-uk-and-protecting-older-people-and-vulnerable-adults .
- Shaker MS ,
- Oppenheimer J ,
- Grayson M ,
- China Medical Treatment Expert Group for Covid-19
- Docherty AB ,
- Harrison EM ,
- Greenhalgh T ,
- Pinnock H ,
- Campbell S ,
- ↵ Scottish Intercollegiate Guidelines Network & British Thoracic Society. Sign 158 British guideline on the management of asthma. 2019. https://www.sign.ac.uk/sign-158-british-guideline-on-the-management-of-asthma .
- ↵ Primary Care Respiratory Society. PCRS Pragmatic Guidance: Diagnosing and managing asthma attacks and people with COPD presenting in crisis during the UK Covid 19 epidemic. 2020. https://www.pcrs-uk.org/sites/pcrs-uk.org/files/resources/COVID19/PCRS-Covid-19-Pragmatic-Guidance-v2-02-April-2020.pdf .
- Rapoport AB
- Royal College of Physicians
- ↵ Centre for Evidence-Based Medicine. Question: Should the Roth score be used in the remote assessment of patients with possible COVID-19? Answer: No. 2020. https://www.cebm.net/covid-19/roth-score-not-recommended-to-assess-breathlessness-over-the-phone/ .
- Goldstein BA ,
- ↵ Public Health England. Guidance: COVID-19 personal protective equipment (PPE). 2020. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control/covid-19-personal-protective-equipment-ppe .
- ↵ British Thoracic Society. Advice for healthcare professionals treating people with asthma (adults) in relation to COVID-19. 2020. https://www.brit-thoracic.org.uk/about-us/covid-19-information-for-the-respiratory-community/ .
- Pauwels RA ,
- Pedersen S ,
- START Investigators Group
- Bestall JB ,
- Lasserson TJ ,
- Spooner C ,
- Ducharme FM ,
- Bretzlaff JA ,
- Spooner CH ,
- Stockman LJ ,
- Bellamy R ,
- ↵ World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance 13th March 2020. 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf .
- ↵ Global Initiative for Asthma (GINA). 2020 GINA report, global strategy for asthma management and prevention. 2020. https://ginasthma.org/gina-reports/ .
- McKeever T ,
- Mortimer K ,
- ↵ Office for National Statistics. Analysis of death registrations not involving coronavirus (COVID-19), England and Wales: 28 December 2019 to 1 May 2020. Release date: 5 June 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/analysisofdeathregistrationsnotinvolvingcoronaviruscovid19englandandwales28december2019to1may2020/technicalannex .

WILLIAM DABBS, MD, MEGAN H. BRADLEY, MD, AND SHAUNTA' M. CHAMBERLIN, PharmD
Am Fam Physician. 2024;109(1):43-50
Author disclosure: No relevant financial relationships.
Asthma exacerbations, defined as a deterioration in baseline symptoms or lung function, cause significant morbidity and mortality. Asthma action plans help patients triage and manage symptoms at home. In patients 12 years and older, home management includes an inhaled corticosteroid/formoterol combination for those who are not using an inhaled corticosteroid/long-acting beta 2 agonist inhaler for maintenance, or a short-acting beta 2 agonist for those using an inhaled corticosteroid/long-acting beta 2 agonist inhaler that does not include formoterol. In children four to 11 years of age, an inhaled corticosteroid/formoterol inhaler, up to eight puffs daily, can be used to reduce the risk of exacerbations and need for oral corticosteroids. In the office setting, it is important to assess exacerbation severity and begin a short-acting beta 2 agonist and oxygen to maintain oxygen saturations, with repeated doses of the short-acting beta 2 agonist every 20 minutes for one hour and oral corticosteroids. Patients with severe exacerbations should be transferred to an acute care facility and treated with oxygen, frequent administration of a short-acting beta 2 agonist, and corticosteroids. The addition of a short-acting muscarinic antagonist and magnesium sulfate infusion has been associated with fewer hospitalizations. Patients needing admission to the hospital require continued monitoring and systemic therapy similar to treatments used in the emergency department. Improvement in symptoms and forced expiratory volume in one second or peak expiratory flow to 60% to 80% of predicted values helps determine appropriateness for discharge. The addition of inhaled corticosteroids, consideration of stepping up asthma maintenance therapy, close follow-up, and education on asthma action plans are important next steps to prevent future exacerbations.
- Immediate, unlimited access to all AFP content
- More than 130 CME credits/year
- AAFP app access
- Print delivery available
Issue Access
- Immediate, unlimited access to this issue's content
- CME credits
Article Only
- Immediate, unlimited access to just this article
The Global Asthma Report 2022. Int J Tuberc Lung Dis. 2022;26(supp 1):1-104.
Centers for Disease Control and Prevention. Most recent national asthma data. Accessed January 17, 2023. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm
McCoy K, Shade DM, Irvin CG, et al.; American Lung Association Asthma Clinical Research Centers. Predicting episodes of poor asthma control in treated patients with asthma. J Allergy Clin Immunol. 2006;118(6):1226-1233.
Meltzer EO, Busse WW, Wenzel SE, et al. Use of the Asthma Control Questionnaire to predict future risk of asthma exacerbation. J Allergy Clin Immunol. 2011;127(1):167-172.
Schatz M, Zeiger RS, Yang SJ, et al. The relationship of asthma impairment determined by psychometric tools to future asthma exacerbations. Chest. 2012;141(1):66-72.
Loymans RJB, Honkoop PJ, Termeer EH, et al. Identifying patients at risk for severe exacerbations of asthma: development and external validation of a multivariable prediction model [published correction appears in Thorax . 2018; 73(8): 795–796]. Thorax. 2016;71(9):838-846.
National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma. 2007. Accessed June 15, 2023. https://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm
Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59-99.
Jackson DJ, Bacharier LB. Inhaled corticosteroids for the prevention of asthma exacerbations. Ann Allergy Asthma Immunol. 2021;127(5):524-529.
Pollart SM, Compton RM, Elward KS. Management of acute asthma exacerbations. Am Fam Physician. 2011;84(1):40-47.
Global Initiative for Asthma. Global strategy for asthma management and prevention. 2023. Accessed June 15, 2023. https://ginasthma.org/wp-content/uploads/2023/07/GINA-2023-Full-report-23_07_06-WMS.pdf
Gibson PG, Powell H. Written action plans for asthma: an evidence-based review of the key components. Thorax. 2004;59(2):94-99.
Abramson MJ, Bailey MJ, Couper FJ, et al.; Victorian Asthma Mortality Study Group. Are asthma medications and management related to deaths from asthma?. Am J Respir Crit Care Med. 2001;163(1):12-18.
Salazar G, Tarwala G, Reznik M. School-based supervised therapy programs to improve asthma outcomes: current perspectives. J Asthma Allergy. 2018;11:205-215.
Cicutto L, To T, Murphy S. A randomized controlled trial of a public health nurse-delivered asthma program to elementary schools. J Sch Health. 2013;83(12):876-884.
Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319(14):1485-1496.
Raymond TJ, Peterson TA, Coulter J. Chronic asthma treatment: common questions and answers. Am Fam Physician. 2023;107(4):358-368.
Cloutier MM, Baptist AP, Blake KV, et al. Expert Panel Working Group of the National Heart, Lung, and Blood Institute administered and coordinated National Asthma Education and Prevention Program Coordinating Committee. 2020 focused updates to the asthma management guidelines [published correction appears in J Allergy Clin Immunol . 2021; 147(4): 1528–1530]. J Allergy Clin Immunol. 2020;146(6):1217-1270.
Szefler SJ. Update on the NAEPPCC asthma guidelines: the wait is over, or is it?. J Allergy Clin Immunol. 2020;146(6):1275-1280.
Kim LHY, Saleh C, Whalen-Browne A, et al. Triple vs dual inhaler therapy and asthma outcomes in moderate to severe asthma: a systematic review and meta-analysis. JAMA. 2021;325(24):2466-2479.
Kew KM, Flemyng E, Quon BS, et al. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database Syst Rev. 2022(9):CD007524.
O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378(20):1865-1876.
Bateman ED, Reddel HK, O’Byrne PM, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378(20):1877-1887.
Crossingham I, Turner S, Ramakrishnan S, et al. Combination fixed-dose beta agonist and steroid inhaler as required for adults or children with mild asthma. Cochrane Database Syst Rev. 2021(5):CD013518.
Nwaru BI, Ekström M, Hasvold P, et al. Overuse of short-acting β 2 -agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872.
Sturdy PM, Victor CR, Anderson HR, et al.; Mortality and Severe Morbidity Working Group of the National Asthma Task Force. Psychological, social and health behaviour risk factors for deaths certified as asthma: a national case-control study. Thorax. 2002;57(12):1034-1039.
Pumphrey RSH, Gowland MH. Further fatal allergic reactions to food in the United Kingdom, 1999–2006. J Allergy Clin Immunol. 2007;119(4):1018-1019.
Alvarez GG, Schulzer M, Jung D, et al. A systematic review of risk factors associated with near-fatal and fatal asthma. Can Respir J. 2005;12(5):265-270.
Chang YL, Ko HK, Lu MS, et al. Independent risk factors for death in patients admitted for asthma exacerbation in Taiwan. NPJ Prim Care Respir Med. 2020;30(1):7.
Roberts G, Patel N, Levi-Schaffer F, et al. Food allergy as a risk factor for life-threatening asthma in childhood: a case-controlled study. J Allergy Clin Immunol. 2003;112(1):168-174.
Nowak RM, Tomlanovich MC, Sarkar DD, et al. Arterial blood gases and pulmonary function testing in acute bronchial asthma. Predicting patient outcomes. JAMA. 1983;249(15):2043-2046.
White CS, Cole RP, Lubetsky HW, et al. Acute asthma. Admission chest radiography in hospitalized adult patients. Chest. 1991;100(1):14-16.
Perrin K, Wijesinghe M, Healy B, et al. Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax. 2011;66(11):937-941.
Rowe BH, Spooner CH, Ducharme FM, et al. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007(3):CD000195.
Edmonds ML, Milan SJ, Camargo CA, et al. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst Rev. 2012(12):CD002308.
Kirkland SW, Vandenberghe C, Voaklander B, et al. Combined inhaled beta-agonist and anticholinergic agents for emergency management in adults with asthma. Cochrane Database Syst Rev. 2017(1):CD001284.
Kew KM, Kirtchuk L, Michell CI. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database Syst Rev. 2014(5):CD010909.
Weber EJ, Silverman RA, Callaham ML, et al. A prospective multicenter study of factors associated with hospital admission among adults with acute asthma. Am J Med. 2002;113(5):371-378.
Grunfeld AF, Fitzgerald JM. Discharge considerations for adult asthmatic patients treated in emergency departments. Can Respir J. 1996;3(5):322-327.
Arnold DH, Gebretsadik T, Minton PA, et al. Assessment of severity measures for acute asthma outcomes: a first step in developing an asthma clinical prediction rule. Am J Emerg Med. 2008;26(4):473-479.
Hasegawa T, Ishihara K, Takakura S, et al. Duration of systemic corticosteroids in the treatment of asthma exacerbation; a randomized study. Intern Med. 2000;39(10):794-797.
Jones AM, Munavvar M, Vail A, et al. Prospective, placebo-controlled trial of 5 vs 10 days of oral prednisolone in acute adult asthma. Respir Med. 2002;96(11):950-954.
Chang AB, Clark R, Sloots TP, et al. A 5- versus 3-day course of oral corticosteroids for children with asthma exacerbations who are not hospitalised: a randomised controlled trial. Med J Aust. 2008;189(6):306-310.
Krishnan JA, Davis SQ, Naureckas ET, et al. An umbrella review: corticosteroid therapy for adults with acute asthma. Am J Med. 2009;122(11):977-991.
Higgins JC. The ‘crashing asthmatic.’. Am Fam Physician. 2003;67(5):997-1004.
Continue Reading
More in AFP
More in pubmed.
Copyright © 2024 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
- Introduction
- Conclusions
- Article Information
AD indicates atopic dermatitis; OCS, oral corticosteroid.
a Rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, psoriasis, Crohn disease, ulcerative colitis, Sjögren syndrome, systemic sclerosis, dermatomyositis, polymyositis, thromboangiitis obliterans, Behçet disease, sarcoidosis, pemphigus, and vitiligo.
b Patients who received a diagnosis of the outcomes of interest (osteoporosis, fracture, type 2 diabetes, hyperlipidemia, hypertension, myocardial infarction, stroke, heart failure, avascular necrosis, cataract, or glaucoma) during 1 year before or 1 year after the cohort entry date were excluded.
OR indicates odds ratio.
a Modified definition of the exposure from cumulative duration of more than 30 days per year and more than 90 days per year to a cumulative duration of more than 60 days per year.
b The long-term use of oral corticosteroids was defined as a cumulative supply of more than 30 days or more than 90 days with a greater than 5-mg daily prednisolone-equivalent dose of oral corticosteroids, which places patients at risk of systemic adverse effects, and we assessed the long-term use of oral corticosteroids annually. To exclude potential use of oral corticosteroids for conditions other than atopic dermatitis, we restricted exposure to prescriptions for patients with a diagnosis of atopic dermatitis.
c Restricted to patients who could be followed up for at least 3 years from the cohort entry date.
d Restricted to patients who could be followed up for at least 5 years from the cohort entry date.
eTable 1. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis for Composite Outcome
eTable 2. Codes Used to Define Exclusion Criteria, Exposures, Outcomes, and Covariates
eTable 3. Exposure Definition Regarding to Long-Term Oral Corticosteroid Usage in the Previous Studies
eTable 4. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis, Comparison Between Ever Long-Term Use of OCS Over 30 Days vs 90 Days
eTable 5. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Osteoporosis
eTable 6. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Fracture
eTable 7. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Type 2 Diabetes Mellitus
eTable 8. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Hyperlipidemia
eTable 9. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Hypertension
eTable 10. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Myocardial Infarction
eTable 11. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Stroke
eTable 12. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Heart Failure
eTable 13. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Avascular Necrosis
eTable 14. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Cataract
eTable 15. Demographic and Clinical Characteristics of Cases and Controls of Adult Patients (>18 Years) With Atopic Dermatitis: Glaucoma
eTable 16. E-Values for Point Estimates of Different Outcomes of Interest for Primary Exposure: >30 Days a Year
eTable 17. E-Values for Point Estimates of Different Outcomes of Interest for Primary Exposure: >90 Days a Year
eFigure 1. Overall Design of This Nested-Case Control Study
eFigure 2. Case-Control Matching Using Risk-Set Sampling Method
eFigure 3 . Explanation for the Exposure Status According to 1) Ever Long-Term OCS, 2) Cumulative No. of Years of Long-Term OCS, 3) Consecutive No. of Years of Long-Term OCS for the Primary (>30 Days) and Secondary (>90 Days) Exposure Definition
eFigure 4. Subgroup Analysis According to the Age Stratification for Evaluating the Risk of Composite Adverse Outcomes Associated With Long-Term Use of OCS
eFigure 5. Subgroup Analysis According to the Sex Stratification for Evaluating the Risk of Composite Adverse Outcomes Associated With Long-Term Use of OCS
eFigure 6. Subgroup Analysis According to the Severity of AD Stratification for Evaluating the Risk of Composite Adverse Outcomes Associated With Long-Term Use of OCS
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Jang YH , Choi E , Lee H, et al. Long-Term Use of Oral Corticosteroids and Safety Outcomes for Patients With Atopic Dermatitis. JAMA Netw Open. 2024;7(7):e2423563. doi:10.1001/jamanetworkopen.2024.23563
Manage citations:
© 2024
- Permissions
Long-Term Use of Oral Corticosteroids and Safety Outcomes for Patients With Atopic Dermatitis
- 1 Department of Dermatology, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea
- 2 School of Pharmacy, Sungkyunkwan University, Suwon, South Korea
- 3 Department of Biohealth Regulatory Science, Sungkyunkwan University, Suwon, South Korea
- 4 Research Department of Practice and Policy, School of Pharmacy, University College London, London, United Kingdom
- 5 School of Pharmacy, Aston University, Birmingham, United Kingdom
- 6 Department of Epidemiology, Biostatistics, and Occupational Health, McGill University, Montreal, Quebec, Canada
- 7 Department of Inflammation & Immunology Medical Affairs, Pfizer Pharmaceuticals Korea Ltd, Seoul, South Korea
- 8 Department of Clinical Research Design & Evaluation, Samsung Advanced Institute for Health Sciences and Technology (SAIHST), Sungkyunkwan University, Seoul, South Korea
- 9 Department of Dermatology, Konkuk University School of Medicine, Seoul, South Korea
Question What duration of oral corticosteroid use is associated with adverse effects among adult patients with atopic dermatitis?
Findings In this nested case-control study including 1 025 270 patients with atopic dermatitis, use of oral corticosteroids for more than 90 days during 1 year was associated with a slightly increased risk of composite adverse outcomes. There was no increased risk with use of oral corticosteroids for more than 30 days.
Meaning This study suggests that for patients with exacerbations of atopic dermatitis, limiting the duration of oral corticosteroid treatment to 90 days or less may limit adverse effects.
Importance The use of oral corticosteroids for prolonged periods may be associated with adverse events (AEs). Nevertheless, the risk of AEs with oral corticosteroids, especially among patients with atopic dermatitis (AD), has not been comprehensively investigated and lacks evidence on duration of treatment.
Objective To assess the association between long-term exposure to oral corticosteroids and AEs among adult patients with AD.
Design, Setting, and Participants This nested case-control study used data from the Health Insurance Review and Assessment Service database of South Korea between January 1, 2012, and October 31, 2021, which included 1 year prior to the cohort entry date of January 1, 2013, for assessing exclusion criteria and baseline characteristics, and 1 year after the study end date of October 31, 2020, to ensure a minimum duration for assessing exposure. Among the population of adults with AD, patients diagnosed with any of 11 AEs were matched with patients who had never received a diagnosis of any of the 11 AEs.
Exposure Long-term use of oral corticosteroids was defined as cumulative supply of more than 30 days or more than 90 days of oral corticosteroid prescription per year.
Main Outcomes and Measures We used multivariable conditional logistic regression analyses to measure the risk of 11 individual outcomes (osteoporosis, fracture, type 2 diabetes, hyperlipidemia, hypertension, myocardial infarction, stroke, heart failure, avascular necrosis, cataract, or glaucoma) as the composite outcome, controlling for potential confounders. We further classified the composite outcome to individual outcomes to evaluate the AE-specific risk.
Results Among 1 025 270 patients with AD between 2013 and 2020, 164 809 cases (mean [SD] age, 39.4 [14.8]; 56.9% women) were matched with 328 303 controls (mean [SD] age, 39.3 [14.7]; 56.9% women) for sex, age, cohort entry date, follow-up duration, and severity of AD, where the balance of most baseline characteristics was achieved. A total of 5533 cases (3.4%) and 10 561 controls (3.2%) were exposed to oral corticosteroids for more than 30 days, while 684 cases (0.4%) and 1153 controls (0.4%) were exposed to oral corticosteroids for more than 90 days. Overall, there was no increased risk of AEs with use of oral corticosteroids for more than 30 days (adjusted odds ratio [AOR], 1.00; 95% CI, 0.97-1.04), whereas the risk was slightly higher with use of oral corticosteroids for more than 90 days (AOR, 1.11; 95% CI, 1.01-1.23). The small elevation in experiencing an AE was observed with each cumulative or consecutive year of ever long-term use.
Conclusions and Relevance This case-control study found a slightly increased risk of AEs associated with use of oral corticosteroids for more than 90 days per year, which warrants future research to fully elucidate the observed findings.
Atopic dermatitis (AD) is a chronic inflammatory disease that causes serious morbidity, such as pruritus, impaired quality of life, and a range of comorbidities. 1 , 2 AD is a lifelong condition that relapses chronically and needs constant care. 3 Although AD is considered primarily a pediatric disease, studies have shown high rates of AD among adults as well. 4 The prevalence of AD among adults ranged from 2.1% to 4.9% across countries, and up to 10% of adults required medication for moderate to severe AD due to inadequate response to topical therapies; the prevalence rates were higher among adult patients than among pediatric patients, of whom 1.5% required medication for moderate to severe AD. 5 - 7
As AD treatment strategies, international guidelines and expert opinions generally recommend that oral corticosteroids should generally be avoided or limited to the short term only as rescue therapy. 8 - 11 Nonetheless, given the benefits of oral corticosteroids, including their effectiveness in allergic diseases, short-term safety, and low cost, many patients with moderate to severe AD are treated with oral corticosteroids for prolonged periods, which may constitute inappropriate or excessive use. 12 , 13 However, oral corticosteroid treatment for prolonged periods could have an association with oral corticosteroid–related complications. 14 Hence, clinical evidence informing patients and practitioners regarding the management of AD exacerbations in routine clinical practice is warranted.
Although previous studies among patients with asthma or rheumatic disease have suggested associations between long-term use of oral corticosteroids and various adverse events (AEs), there are few studies of patients with AD, to our knowledge. 15 - 21 In addition, existing studies about corticosteroid use among patients with AD were conducted to evaluate the safety concerns primarily about topical corticosteroids. 22 - 29 Considering the frequent use of oral corticosteroids among adults with AD and the potential association between long-term use of oral corticosteroids and AEs, some of which are severe, there is a need to investigate the safety of the long-term use of oral corticosteroids among adults with AD. 6 , 30 , 31 Accordingly, we aimed to investigate the association between long-term use of oral corticosteroids and AEs among adult patients with AD in South Korea.
We used the nationwide Health Insurance Review and Assessment Service (HIRA) database of South Korea between January 1, 2012, and October 31, 2021, which included 1 year prior to the cohort entry date of January 1, 2013, for assessing exclusion criteria and baseline characteristics, and 1 year after the study end date of October 31, 2020, to ensure a minimum duration for assessing exposure. It encompasses comprehensive data on health care use for every resident of South Korea, ensuring that patient identifiers are anonymized. The database collects information on socioeconomic and demographic variables, diagnosis ( International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnostic code; setting of diagnosis; date of diagnosis; and others), and medications prescribed (national drug chemical code, days’ supply, dose, date of prescription, route of administration, and others) until the occurrence of emigration or death. A prior validation study examined diagnosis codes documented in the HIRA in comparison with those in electronic medical records and found an overall positive predictive value of 82.3%. 32 This study was approved by the institutional review board of Sungkyunkwan University, which waived the informed consent because only deidentified data were used in this study. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline. 33
The study cohort comprised patients who were prescribed oral corticosteroids at least once with an AD diagnosis code from January 1, 2013, to October 31, 2020. The cohort entry date was defined as the first date of the prescription of oral corticosteroids with an AD diagnosis within the study period to include the new users of oral corticosteroids. Eligible case and control groups were identified after excluding the following: (1) patients with a diagnosis of immune-mediated inflammatory diseases during a 1-year window of exclusion assessment before the cohort entry date, to evaluate the risk of AEs from oral corticosteroid use for AD; (2) patients with a diagnosis of any of 11 outcomes of interest during the exclusion assessment window of 1 year before and 1 year after the cohort entry date, to investigate the association of oral corticosteroid use with newly occurred outcomes; and (3) patients who were younger than 18 years of age on the cohort entry date, to include adult patients ( Figure 1 ).
Cases were defined as patients with AD who received a diagnosis of any of our outcomes of interest after the cohort entry date, and the index date was defined as the first date of outcome occurrence. The composite outcome of interest consisted of osteoporosis, fracture, type 2 diabetes, hyperlipidemia, hypertension, myocardial infarction, stroke, heart failure, avascular necrosis (AVN), cataract, and glaucoma. We defined controls as patients with AD who never received a diagnosis of our outcomes of interests after the cohort entry date. We matched each case with up to 2 controls without replacement, using risk-set sampling on the cohort entry date (±30 days), follow-up duration (between the cohort entry date and the index date [±30 days]), age, sex, and severity of AD. Disease severity of AD was classified as moderate to severe on the basis of the current treatment guidelines for AD. 7 Moderate to severe AD was defined as patients who were receiving at least 1 immunosuppressant, alitretinoin, intravenous immunoglobulin, dupilumab, or phototherapy during the 1 year prior to the cohort entry date. The index dates of the control group were aligned with the corresponding index date of their respective matched cases. For individual outcomes, each case was matched with up to 5 or 10 controls, using different numbers from the composite outcome for ensuring statistical power according to the size of cases for each outcome variable, using risk-set sampling as well (eFigure 2 in Supplement 1 ).
We defined the exposure ascertainment window as the period between the cohort entry date and the index date, segmenting the period into yearly intervals to assess exposure year by year to determine whether patients met the definition for long-term use of oral corticosteroids. Owing to the absence of consensus for a definition of long-term oral corticosteroid use among patients with AD, and even for other diseases, we set the classification of long-term oral corticosteroid use as follows: cumulatively more than 30 days as the primary definition for modest long-term use or more than 90 days as a secondary definition for extensive long-term use, both with greater than a 5-mg daily prednisolone-equivalent dose of oral corticosteroids per year, which places patients at risk of systemic adverse effects. 17 To exclude potential use of oral corticosteroids for related conditions other than AD, we restricted exposure to prescriptions of oral corticosteroids to patients with a diagnosis of AD. Ever long-term use was defined as patients with a history of long-term use of oral corticosteroids for at least 1 year, and all remaining patients were defined as no long-term use . Primarily, ever long-term use was defined as a binary variable using 2 thresholds (>30 days and >90 days). In addition, to examine the duration-response association with long-term use of oral corticosteroids, we used the year, which met the definition for the long-term use, as a continuous variable. We assessed the risk of each outcome associated with the number of cumulative years (considering all the intermittent years of long-term use of oral corticosteroids) throughout the exposure ascertainment period. We also evaluated the risk associated with the number of consecutive years (considering only the continuous years of long-term use of oral corticosteroids) within the exposure ascertainment period. Details of the exposure assessment are shown in eFigure 3 in Supplement 1 .
We discerned a sufficient collection of confounding variables that adequately accounted for potential biases in our analysis: demographic characteristics (eg, sex, age, and medical aid recipients), comorbidities (eg, allergic rhinitis, depression, chronic obstructive pulmonary disease, and thyroid disorders), comedications (eg, antidepressants, antibiotics, estrogens, and proton-pump inhibitors), proxies of overall health status (eg, history of hospitalization, number of outpatient visits, and Charlson Comorbidity Index score), and severity of AD. The characteristic assessment window was defined as the 1-year period before the cohort entry date (eFigure 1 in Supplement 1 ; the demographic characteristics [sex, age, insurance] were assessed on the cohort entry date and other characteristics such as comorbidities, comedications, proxies of health status, and severity of AD during the 1 year prior to cohort entry). The details of exclusion criteria, exposures, outcomes, and covariates are presented in eTable 2 in Supplement 1 .
The demographic characteristics of the cases and controls were presented as frequency (proportion) for categorical variables and as mean (SD) or median (IQR) values for continuous variables. The same analysis used to evaluate the demographic characteristics of the cases and controls of patients with AD were repeated for each of the 11 outcomes as secondary outcomes. Differences in baseline covariates between cases and controls were evaluated using the absolute standardized difference, where an absolute standardized difference greater than 0.1 indicates a statistical imbalance existing between 2 groups.
The association between long-term oral corticosteroid use and the risk of the composite and individual outcomes were investigated using multivariable conditional logistic regression analyses to estimate adjusted odds ratios (AORs) with 95% CIs, adjusting for unbalanced comorbidities, comedications, and proxies of health status after the matching. We conducted additional analyses by considering the number of cumulative or consecutive years of long-term use of oral corticosteroids throughout the entire exposure ascertainment window as continuous variables, to investigate the monotonic duration-response association.
The potential heterogeneity of long-term treatment adverse effects in selected subgroups of patients with AD was examined for the composite adverse outcomes according to age (18-39, 40-64, and ≥65 years), sex (male or female), and severity of AD (mild or moderate to severe AD). To evaluate the robustness of the main findings, sensitivity analyses were first conducted by modifying the definition of exposure from a cumulative duration of more than 30 days or more than 90 days per year to more than 60 days per year. Second, we restricted the population to patients who could be followed up for at least 3 years or 5 years from the cohort entry date. All statistical tests were 2 sided. Analyses were conducted using SAS Enterprise Guide, version 7.1 (SAS Institute Inc), provided by HIRA through a virtual access machine.
Of 1 025 270 patients with AD who had at least 1 prescription of oral corticosteroids between 2013 and 2020, we matched 164 809 cases (mean [SD] age, 39.4 [14.8]; 56.9% women and 43.1% men) with 328 303 controls (mean [SD] age, 39.3 [14.7]; 56.9% women and 43.1% men) ( Table 1 ) by 1:2 matching using risk-set sampling. Cases and controls were matched for sex, age, cohort entry date, follow-up duration, and severity of AD; balance was achieved for most covariates between the 2 groups, with an absolute standardized difference of less than 0.1 ( Table 1 ; whole baseline characteristics of cases and controls are presented in eTable 1 in Supplement 1 , individual outcomes in eTables 5-15 in Supplement 1 , and modest long-term [>30 days] vs extensive long-term [>90 days] in eTable 4 in Supplement 1 ). The most common comorbidity was allergic rhinitis (cases, 42.2%; controls, 38.7%), and the most prevalently prescribed concurrent medication was antibiotics (cases, 71.3% and controls, 66.8%). All the imbalanced variables of concurrent medication use and number of outpatient visits were additionally adjusted in the multivariable logistic regression.
Among the 164 809 cases and 328 303 controls, 5533 cases (3.4%) and 10 561 controls (3.2%) were exposed to oral corticosteroids over 30 days, and 684 cases (0.4%) and 1153 controls (0.4%) were exposed to oral corticosteroids over 90 days. Overall, the risk of AEs was not associated with use of oral corticosteroids exceeding 30 days (AOR, 1.00; 95% CI, 0.97-1.04) ( Table 2 ), while use of oral corticosteroids exceeding 90 days was associated with an 11% increased risk of the composite adverse outcome (AOR, 1.11; 95% CI, 1.01-1.23) ( Table 3 ). Each cumulative or consecutive additive year of long-term exposure (>90 days a year) was associated with a slightly increased risk of having an AE (AOR, 1.06; 95% CI, 1.00-1.13 and AOR, 1.06; 95% CI, 1.00-1.13, respectively).
In the analyses of individual outcomes, an increased risk for hypertension (AOR, 1.09; 95% CI, 1.03-1.15), AVN (AOR, 2.56; 95% CI, 1.82-3.62), and cataract (AOR, 3.22; 95% CI, 1.05-9.85) was associated with use of oral corticosteroids for more than 30 days ( Table 2 ). An increased risk for fracture (AOR, 1.22; 95% CI, 1.05-1.42), hyperlipidemia (AOR, 1.16; 95% CI, 1.03-1.30), myocardial infarction (AOR, 2.22; 95% CI, 1.17-4.22), and AVN (AOR, 6.88; 95% CI, 3.53-13.42) was associated with use of oral corticosteroids for more than 90 days ( Table 3 ). In our subgroup analysis, as compared with unexposed patients, the risk of composite AEs associated with long-term use of oral corticosteroids was generally consistent with the main analyses. No differences were observed in the stratified analyses according to the age group, sex, and severity of AD (eFigures 4-6 in Supplement 1 ). Furthermore, the results of composite outcomes demonstrated a high degree of consistency across all sensitivity analyses regarding the point estimates ( Figure 2 ).
We identified 164 809 cases and 328 303 controls of comparable patients with AD. The risk of composite adverse outcomes was not associated with with ever long-term use of oral corticosteroids exceeding 30 days, whereas the risk was slightly associated with ever long-term use exceeding 90 days. Also, the cumulative and consecutive years of ever long-term use throughout entire exposure ascertainment period was associated with a monotonic elevated risk of having an AE, although there was not a large discrepancy between the 2 distinctive analyses of additive years. Furthermore, small increased risks were identified in the examination of individual outcomes of fracture, hyperlipidemia, hypertension, myocardial infarction, AVN, and cataract. Generally consistent findings, with regard to point estimates, were observed across a range of sensitivity analyses.
Considering the overlapping pathogenetic mechanism between AD and asthma, we referred to studies of patients with asthma for comparison. One cohort study using Medicaid data found that the use of medium and high doses of systemic corticosteroids was associated with bone, cardiovascular, metabolic, and ocular AEs. 34 Another cohort study using 2000-2014 MarketScan data showed a similar increased risk of various AEs associated with the use of 1 to 3 oral corticosteroid prescriptions (AOR, 1.04; 95% CI, 1.01-1.06) and the use of 4 or more prescriptions (AOR, 1.29; 95% CI, 1.20-1.37); the cumulative burden also increased as the number of years accumulated. 20 Although previous research evaluated the frequency of oral corticosteroid use based on prescription numbers, our study provided more conclusive and valid clinical evidence by defining long-term use based on exact duration.
For individual outcomes, in line with previous studies, we also identified fracture, hypertension, hyperlipidemia, and myocardial infarction as AEs associated with long-term use of oral corticosteroids, owing to interruption of endocrine function and metabolism. 20 , 35 - 38 We observed risks of AVN and cataract with long-term oral corticosteroid use, although the risks of these 2 conditions were inconclusive in past studies. For the underlying mechanisms for AVN of the femoral head, the use of oral corticosteroids leads to intravascular coagulation that results in a inhibition of blood flow to the bones, which consequently triggers ischemic injury. 39 - 41 Although existing evidence regarding an association of AVN with duration of oral corticosteroid treatment is unclear, AVN could be induced from use of just over 30 days, and cumulative exposure is the important determining factor, as shown in our results. 39 Furthermore, although a complete elucidation remains uncertain, the mechanisms of new-onset cataract associated with modest long-term use of oral corticosteroids may be due to disturbances in osmotic equilibrium, oxidative detriment, and perturbations in lens growth factors. 42 , 43 Another potential hypothesis involves nonenzymatic Schiff base intermediates that form between the corticosteroid’s C-20 ketone group and its nucleophilic groups, undergoing Heyns rearrangement to produce stable amine-substituted adducts seen only in corticosteroid-induced posterior subcapsular cataracts. 44 , 45 No association or subtle increased hazard was observed with osteoporosis, glaucoma, stroke, or heart failure, implying that the dose and duration of corticosteroid treatment may not pose a risk for these conditions among patients with AD.
This study has some strengths. Concerns about conducting this study arose from the lack of consensus regarding the definition of long-term corticosteroid treatment, as different criteria have been used and variations have been observed (eTable 3 in Supplement 1 ). Accordingly, we combined the NICE (National Institute for Health and Care Excellence) guidelines 17 with the opinions of clinicians practicing in clinical settings. Even though evidence for a safe continuous duration of corticosteroid treatment was not available as we developed criteria for the definition of long-term treatment for the dichotomous variable, our criteria are expected to serve as a primary threshold for deciding the duration of treatment. In addition, although the long-term use of oral corticosteroids is not recommended in the guideline for treatment of AD, relatively prolonged use of oral corticosteroids is identified frequently in clinical practice. 12 Thus, this study addresses a significant gap in research by investigating the association between long-term oral corticosteroid use and a comprehensive range of AEs specifically among adults with AD. With its substantial sample size, the study provides robust statistical power to detect associations between oral corticosteroid use and relatively rare outcomes, adding to the existing evidence.
This study also has some limitations. First, disparities arose between the diagnoses recorded and the actual diseases a patient had. 46 In addition, HIRA data do not include clinical data; accordingly, the diagnostic standard criteria for AD, such as the Hanifin-Rajka criteria, 47 , 48 were infeasible. To comply with this issue, we included patients with AD who had at least 1 oral corticosteroid prescription and restricted prescriptions to patients with a diagnosis of AD. Second, due to the inbuilt characteristics of database recording drugs that are prescribed rather than drugs that are taken, the exposure measurement could be uncertain. However, we set the exposed group from the modest long term (>30 days) to the extensive long term (>90 days) and also included the numbers of cumulative or consecutive years of ever long-term use, from which the cumulative burden would be appropriately measured. Third, inhaled corticosteroids, which have some degree of systemic bioavailability, and topical and eye drop formulations of corticosteroids were not accounted for in this study. Fourth, for some of the individual study outcomes, we could not rule out the failure to detect the true effect due to the lack of statistical power; thus, future studies are warranted to corroborate these results. Fifth, due to the nature of the case-control design, it is not possible to completely exclude reverse causality. Sixth, although we considered moderate to severe AD using prescriptions of medication based on the treatment guideline, the influence of AD-related disease severity cannot be eliminated. Seventh, we addressed residual or unmeasured confounders by calculating E-values (eTables 16 and 17 in Supplement 1 ), but unmeasured confounders may be present, and the results should be interpreted with caution.
In this large population-based case-control study, we discovered that oral corticosteroid use of more than 90 days among individuals with AD was associated with a small increased risk of composite adverse outcomes. Future investigations are warranted to confirm this potential risk of AEs associated with long-term use of oral corticosteroids for patients with exacerbations of AD, and health care professionals should thoroughly weigh the benefits associated with oral corticosteroids against the observed small risk of AEs, while continuously monitoring for AEs.
Accepted for Publication: May 23, 2024.
Published: July 19, 2024. doi:10.1001/jamanetworkopen.2024.23563
Open Access: This is an open access article distributed under the terms of the CC-BY-NC-ND License . © 2024 Jang YH et al. JAMA Network Open .
Corresponding Authors: Ju-Young Shin, PhD, School of Pharmacy, Sungkyunkwan University, 2066 Saburo, Jangan-gu, Suwon, Gyeonggi-do 16419, South Korea ( [email protected] ); Yang Won Lee, MD, PhD, Department of Dermatology, Konkuk University School of Medicine, 120-1 Neungdong-ro, Gwangjin-gu, Seoul 05030, South Korea ( [email protected] ).
Author Contributions: Drs Shin and Y. W. Lee had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Jang and Choi contributed equally to this work.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: H. Lee, Noh, Jeon, Yoo.
Drafting of the manuscript: Jang, Choi, Woo, Jeon, Yoo.
Critical review of the manuscript for important intellectual content: Jang, H. Lee, Woo, Park, Noh, Jeon, Yoo, Shin, Y. W. Lee.
Statistical analysis: Choi, H. Lee, Woo, Park.
Obtained funding: Jeon, Yoo, Shin.
Administrative, technical, or material support: Jeon, Yoo, Shin, Y. W. Lee.
Supervision: Jang, Shin.
Conflict of Interest Disclosures: Dr Park reported receiving support from the AIR@innoHK programme of the Government of Hong Kong Special Administrative Region Innovation and Technology Commission. Dr Noh reported receiving grants from the Ministry of Health and Welfare outside the submitted work. Drs Jeon and Yoo reported receiving personal fees from Pfizer Pharmaceuticals Korea Ltd outside the submitted work. Dr Shin reported receiving grants from the Ministry of Food and Drug Safety, the Ministry of Health and Welfare, the National Research Foundation of Korea, Celltrion, and SK Bioscience outside the submitted work. No other disclosures were reported.
Funding/Support: This work was supported by Pfizer Pharmaceuticals Korea Ltd.
Role of the Funder/Sponsor: Pfizer Pharmaceuticals Korea Ltd had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Short report
- Open access
- Published: 30 August 2024
Exploratory pharmacodynamics and efficacy of PF-06817024 in a Phase 1 study of patients with chronic rhinosinusitis and atopic dermatitis
- Spencer I. Danto ORCID: orcid.org/0000-0002-0587-5274 1 na1 ,
- Nikolaos Tsamandouras 1 ,
- Padma Reddy 1 ,
- Steven A. Gilbert 1 ,
- Jessica Y. Mancuso 1 ,
- Karen Page 1 ,
- Jean S. Beebe 1 ,
- Elena Peeva 1 &
- Michael S. Vincent 1
Allergy, Asthma & Clinical Immunology volume 20 , Article number: 46 ( 2024 ) Cite this article
Metrics details
PF-06817024 is a humanized antibody against interleukin-33 that has the potential to inhibit type 2 inflammation. An exploratory analysis of the pharmacodynamics and clinical effects of single and repeat doses of PF-06817024 was assessed in patients with chronic rhinosinusitis with nasal polyps (CRSwNP) and patients with moderate-to-severe atopic dermatitis (AD), respectively, as part of a Phase 1, first-in-human study. Rhinosinusitis symptoms were improved, and nasal polyps were decreased in size following treatment with PF-06817024 in patients with CRSwNP. In patients with AD, PF-06817024, in aggregate, reduced disease severity and improved symptoms, as demonstrated by greater percentage decrease from baseline in Eczema Area and Severity Index (EASI) scores and reduced pruritus numerical rating scores, compared with placebo. The efficacy in AD appeared to be bimodal with a sub-group of participants exhibiting high levels of improvement (EASI75 and EASI90) for a sustained period of time after dosing. In patients with CRSwNP, a consistent trend of decrease in eosinophil levels was observed in the PF-06817024 group, compared with placebo. Further research would be needed to confirm the clinical benefit and safety of PF-06817024 as a treatment for allergic diseases.
Trial registration ClinicalTrials.gov, NCT02743871. Registered 15 April 2016, https://clinicaltrials.gov/study/NCT02743871?term=NCT02743871&rank=1 .
Introduction
Allergic diseases such as asthma, allergic rhinitis, and atopic dermatitis (AD) represent major public health challenges, with the global prevalence of such diseases increasing each year [ 1 , 2 ]. Chronic rhinosinusitis with nasal polyps (CRSwNP) is an inflammatory disease with a reported prevalence ranging from 1.0 to 2.6% [ 3 ]. Common symptoms include sinus pressure, nasal congestion, and a decreased sense of smell [ 4 ], which cause patients with CRSwNP to have a lower quality of life [ 5 ]. AD is a chronic inflammatory skin disease that typically starts in infancy, with reported prevalence in children ranging from 2.7 to 20.1%; however, it is also highly prevalent in adults, with a range of 2.1–4.9% [ 6 , 7 , 8 ]. Clinical manifestations of AD vary with age, with the scalp, face, neck, and trunk affected in infants, while in children and adolescents the flexural surfaces of the extremities are typically more affected [ 9 ]. AD significantly impairs quality of life as the disease is associated with anxiety, depression, and sleep disturbances [ 10 , 11 ].
Type 2 inflammation has been implicated in the pathophysiology of atopic diseases such as CRSwNP and AD. The type 2 inflammatory response is mediated, in part, by T helper 2 (T H 2) cells and type 2 innate lymphoid cells (ILC2s) [ 12 ]. Following exposure to an allergen, damaged epithelial cells release alarmins, including interleukin (IL)-33 [ 13 ]. IL-33 binds to its receptor, suppression of tumorigenicity 2 (ST2), which is primarily located on mast cells and T H 2 cells, and triggers the production of T H 2 cytokines such as IL-4, IL-5, IL-13, and IL-31 [ 14 , 15 ]. These cytokines contribute to the pathological hallmarks of allergic diseases; IL-4 and IL-13 are involved in immunoglobulin E (IgE) class switching in B cells, while IL-5 induces the production and survival of eosinophils [ 12 ]. In patients with moderate-to-severe AD, the onset of acute lesions was associated with significant increases in gene expression levels of IL-4 and IL-13 [ 16 ]. In addition, samples obtained from patients with CRSwNP also showed high levels of T H 2 cytokines, including IL-4, IL-5, and IL-13 [ 17 ]. Since IL-33 drives the production of such cytokines, its blockade represents a promising therapeutic approach for both CRSwNP and AD. PF-06817024 is a humanized antibody against IL-33 that prevents IL-33 from binding to ST2, and thus triggering a type 2 inflammatory response that is characteristic of CRSwNP and AD.
This paper presents the results from an exploratory analysis of the pharmacodynamics (PD) and clinical effects of single or repeat doses of PF-06817024 in patients with CRSwNP and patients with moderate-to-severe AD, respectively, in the context of a first-in-human clinical study. The safety, tolerability, pharmacokinetics (PK), and immunogenicity of PF-06817024 in these populations were reported separately along with data from single and repeat doses in healthy volunteers.
Study design
The full study design is described in the companion publication of safety, tolerability, PK, and PD [ 18 ]. Briefly, the assessment of clinical effect and biomarkers of PF-06817024 were exploratory endpoints in a Phase 1, randomized, double-blind, placebo-controlled study that assessed the safety, tolerability, PK, and immunogenicity of PF-06817024 in healthy participants (Part 1), participants with CRSwNP (Part 2), and participants with AD (Part 3) (ClinicalTrials.gov, NCT02743871). The current brief report describes the results of exploratory analyses of signs of the clinical effect of PF-06817024 in participants with CRSwNP and AD. In Part 2 of the study, participants with CRSwNP were randomized (1:1) to receive a single intravenous (IV) dose of PF-06817024 300 mg or placebo. Participants were followed for > 211 days after the single dose, divided into a treatment period of 2 days, a follow-up period of 211 days, and an extended follow-up period thereafter, defined by the PK profile. In Part 3 of the study, participants with moderate-to-severe AD were randomized to receive repeat doses of PF-06817024 or placebo at a ratio of 2:1, although actual recruitment was closer to a ratio of 3:1, resulting in a mixed randomization ratio. The dosing regimen in Part 3 consisted of a single 600 mg IV loading dose, followed by three IV doses of 300 mg every 4 weeks. This dosing regimen was guided by emerging total IL-33 clinical data suggesting that higher and more frequent (monthly) dosing may be needed clinically than that predicted by in vitro antibody affinity assays for continuous, high level, suppression of IL-33 levels; a detailed rationale of the dosing regimens has been provided previously [ 18 ]. The defined treatment period was 113 days, with a standard follow-up period of 337 days, and an extended follow-up period thereafter. The extended follow-up period for both parts of the study was necessitated by the extended half-life of PF-06817024 (83–94 days) observed in Parts 1 and 2 of the study. For all parts of this study, blood samples were collected before dosing and at time points specific for the participant cohort as described previously [ 18 ]. All parts of this study were participant- and investigator-blinded.
The study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Council for Harmonisation of Good Clinical Practice Guidelines.
Participants
Participants aged 18–65 years with a body mass index (BMI) of 17.5–35 kg/m 2 and a total body weight of > 50 kg with a history of CRSwNP were eligible for inclusion if they had: a minimum bilateral nasal polyp score (NPS) of 5 out of a maximum score of 8 and the presence of at least two of the following symptoms prior to screening: nasal blockade/obstruction/congestion, nasal discharge, facial pain/pressure, and reduction or loss of smell; were otherwise healthy (comorbid, controlled asthma with a forced expiratory volume in 1 s [FEV 1 ] > 60% predicted was permitted). Participants were excluded from the study if they had received anti-IgE or anti-IL-5 therapy within 130 days prior to screening, had a 22-item Sino-nasal Outcome Test (SNOT-22) score < 7, or had undergone any nasal surgery within 6 months before screening.
Participants aged between 18 and 75 years with a BMI of 17.5–40 kg/m 2 and a total body weight of > 50 kg were eligible for inclusion if they had: a clinical diagnosis of chronic AD (for at least 1 year prior to Day 1) with an inadequate response to treatment with topical medications; moderate-to-severe AD, defined as having an affected body surface area (BSA) ≥ 10%, investigator global assessment (IGA) ≥ 3, and Eczema Area and Severity Index (EASI) score ≥ 12 at screening and baseline visits; were otherwise healthy (comorbid, controlled asthma with an FEV 1 > 60% predicted was permitted). Participants were excluded if they had evidence of skin conditions such as psoriasis, seborrheic dermatitis, or lupus; had received systemic corticosteroids within 4 weeks prior to the first dose of the study drug; or had received dupilumab within 4 months of the first dose of the study drug or another anti-IL-4 and/or anti-IL-13 targeted therapies within 6 months of the first dose of the study drug.
The pharmacologic effect of PF-06817024 was assessed by measuring the change from baseline in several serum biomarkers, including IL-5, IgE, chemokine (C-C motif) ligand 17 (CCL17), chemokine (C-C motif) ligand 26 (CCL26), circulating eosinophils, basophils, and ILC2s in participants with CRSwNP; and IgE, CCL17, and high-sensitivity C-reactive protein (hsCRP) in patients with AD.
In addition, exploratory measures of clinical effect of the single dose and multiple doses of PF-06817024 were also assessed in the CRSwNP and AD patient cohorts, respectively. In participants with CRSwNP, Lund-Mackay computerized axial tomography (CT) score [ 19 ], NPS [ 20 ], University of Pennsylvania Smell Identification Test (UPSIT) [ 21 ], the SNOT-22 score [ 22 ], and 5-item version of the Asthma Control Questionnaire (ACQ-5; asthmatic patients only) [ 23 ] were monitored. In participants with AD, the percentage change from baseline in the total EASI score, EASI50/75/90, affected BSA, IGA, and Scoring Atopic Dermatitis (SCORAD) were monitored. The change from baseline in patient-reported outcomes (PROs), including pruritus numerical rating scale, patient-oriented eczema measure, Dermatology Life Quality Index, Hospital and Anxiety Depression Scale, and ACQ-5 (asthmatic patients only) were also assessed.
Statistical analyses
Statistical methods.
All efficacy data were listed and summarized separately by part, and biomarker data were summarized descriptively. For Part 2 in patients with CRSwNP, as these were exploratory endpoints, no formal hypothesis testing was performed in this study. However, post-hoc efficacy and biomarker analyses were conducted, with least squares means (LSMs) and 80% confidence intervals (CIs) calculated based on analysis of covariance with independent variable of treatment groups and baseline result. Binary variables were summarized by the number of responders and the number of participants with data.
In Part 3, a mixed model for repeated measures (MMRM) analysis of change from baseline and percentage change from baseline in EASI scores was performed, with LSMs and 90% CIs presented for Day 113, the pre-specified landmark time point for assessment of clinical effect. The model included percentage change from baseline as the dependent variable and baseline EASI, treatment, study day, inverse study day (1/study day), treatment by study day, and treatment by inverse study day as fixed effects. Study day and inverse study day were included as continuous variables (not as class variables) and used the observed day (not the scheduled day). The MMRM analyses were performed in R using the lme package and included a random subject effect and a first-order autoregressive correlation over time. To understand the data generated by the pre-specified statistical analyses, additional post-hoc analyses were conducted.
In total, 20 participants with CRSwNP were randomized (11 in the PF-06817024 group and nine in the placebo group). A majority of randomized participants were male (65.0%), with a mean (standard deviation [SD]) age of 54.4 (6.2) years in the PF-06817024 group and 42.8 (10.7) years in the placebo group. Four participants with CRSwNP discontinued from the study: one participant in the PF-06817024 group discontinued during the extended follow-up period (Day 298); three participants in the placebo group discontinued during the extended follow-up period (> 211).
There was no consistent difference in change from baseline in Lund-Mackay CT score (mean [SD]: -5.1 [26.41]) in the PF-06817024 group, compared with placebo [(1.8 [31.43]). Generally, numerically greater decreases from baseline in NPS were observed during the follow-up period (Days 32–181) in the PF-06817024 group (-1.5 [1.92]) than in the placebo groups (-0.4 [1.13]). Increases from baseline in UPSIT scores were observed during the follow-up period (Days 61–211) in the PF-06817024 group (6.9 [10.14]) compared with decreases in the placebo group (-5.4 [11.41]), suggesting improved olfactory function in patients who received PF-06817024. Greater decreases from baseline in SNOT-22 scores were also observed in the PF-06817024 group (-13.5 [15.96]) compared with the placebo group (-5.7 [9.38]). In participants with comorbid asthma, lower ACQ-5 scores were observed in participants in the PF-06817024 group (-2.0 [2.83]) compared with participants in the placebo group (2.1 [7.20]) during the follow-up visits, suggesting improved asthma control. The clinical and PRO results suggest potential shrinkage of nasal polyps and modest improvement in symptoms and social and emotional consequences, respectively, and are suggestive of some beneficial effect of PF-06817024.
For efficacy and PRO endpoints, data from Day 61 are shown in Table 1 , and the percentage change from baseline in efficacy endpoints can be seen in Fig. 1 .
Percentage change from baseline in efficacy endpoints at Day 61 in patients with CRSwNP (placebo-corrected) a
Note: baseline is defined as the last measurement prior to first dosing. Circle represents LSM of percentage change from baseline in comparing with placebo. The estimate and CI were calculated based on ANCOVA analysis with independent variable of treatment groups and baseline result. Only asthmatic patients completed the ACQ-5. NPS data shown are for bilateral score. Lund-Mackay CT score was assessed by two expert reviewers; scores shown here are those from expert reviewer 1. Bars represent 80% CIs
a n =11 for UPSIT, SNOT-22, NPS, and Lund-Mackay CT score in the PF-06817024 group; n = 7 for ACQ-5 in the PF-06817024 group; n = 9 for UPSIT, SNOT-22, NPS, and Lund-Mackay CT score in the placebo group; and n = 7 for ACQ-5 in the placebo group
ACQ-5, 5-item version of the Asthma Control Questionnaire; ANCOVA, analysis of covariance; CI, confidence interval; CRSwNP, chronic rhinosinusitis with nasal polyps; CT, computerized axial tomography; LSM, least squares mean; NPS, nasal polyp score; SNOT-22, 22-item Sino-nasal Outcome Test;UPSIT, University of Pennsylvania Smell Identification Test
Decreases from baseline in blood eosinophil levels were observed at Day 61 with clear separation between PF-06817024 and placebo. However, there were no clear differences from placebo in other circulating biomarkers such as IL-5, IgE, CCL17, CCL26, ILC2s, and basophils at Day 61. LSMs, and associated 80% CIs, of percentage change from baseline for biomarkers at Day 61 are presented in Fig. S1 A.
Overall, 28 patients with AD were randomized and treated (20 in the PF-06817024 group and eight in the placebo group). The randomized patients had a mean (SD) age of 38.9 (13.8) years in the PF-06817024 group and 41.0 (17.4) years in the placebo group, and 32.1% were male. There were 22 patients who discontinued: four in the PF-06817024 group and three in the placebo group during the treatment period; two patients each in the PF-06817024 and placebo groups during follow-up; nine in the PF-06817024 group and two in the placebo group during the extended follow-up period. The most common reason for discontinuation was “no longer willing to participate.”
A greater percentage decrease from baseline in total continuous mean EASI scores was observed in the PF-06817024 group, compared with placebo at Day 113, the pre-specified time point for assessment of clinical effect (Fig. 2 ). The LSM (90% CI) percentage change from baseline in EASI scores was − 60.4 (-71.9, -48.9) and − 16.2 (-34.5, 2.1) in the PF-06817024 and placebo group, respectively, at Day 113 (Table 2 ). In addition, a higher proportion of patients achieved a 50%, 75%, and 90% reduction in EASI score in the PF-06817024 group compared with the placebo group (Table 2 ), with responses observed up to Day 113. EASI scores continued to improve during the follow-up period (up to Day 337) and for a subset of the participants that remained in the study, a robust response was maintained even during extended follow-up (beyond Day 337; Fig. S2 ). Interestingly, post-hoc analyses of the individual responses indicated that the mean effect reflected the average of a bimodal/variable response pattern in which some patients experienced no response following administration of PF-06817024 at all timepoints, while others demonstrated high levels of improvement (75–90% EASI responses) (Fig. S3 ).

Longitudinal percentage change from baseline in EASI scores in patients with AD
Note: baseline is defined as the last measurement prior to the first dosing. MMRM contains fixed factors of baseline EASI, treatment, study day, inverse study day, treatment by study day, and treatment by inverse study day and random factor of subject
AD, atopic dermatitis; CI, confidence interval; EASI, Eczema Area and Severity Index; IV, intravenous; LSM, least squares mean; MMRM, mixed model repeated measures
There were no clear separations from placebo observed in IgE, CCL17, and hsCRP in patients with AD. LSM (and associated 80% CIs) data of percentage change from baseline for biomarkers at Day 113 are presented in Figure S1 B.
Signs of clinical effect of PF-06817024 in patients with CRSwNP and AD were assessed as exploratory endpoints in a Phase 1, placebo-controlled, first-in-human study. Treatment with PF-06817024 led to a consistent improvement of symptoms in Part 2 of the study, while patients with AD also reported reduced disease severity, compared with those who received placebo.
IL-33 plays an important role in amplifying type 2 immune responses via its action on multiple target cells, including basophils, eosinophils, and ILC2s [ 24 ], and induction of cytokines such as IL-4, IL-5, and IL-13 [ 25 , 26 ]. In patients with CRSwNP, IL-5, IgE, CCL17, CCL26, ILC2s, and circulating eosinophils and basophils were measured, while in patients with AD, IgE, CCL17, and hsCRP were monitored to assess any PF-06817024-mediated effect on IL-33 downstream activities. However, one limitation of this study was that biomarkers, such as cytokines and ILC2s, were not assessed in the more disease-relevant mucosal tissues where treatment-related changes may have been more prominent. A single dose of PF-06817024 in patients with CRSwNP was associated with reductions in circulating eosinophils, a biomarker that reflects circulating IL-5 activity [ 27 ]. Similar reductions in eosinophils were reported in a previous Phase 1 study with the IL-33 inhibitor itepekimab [ 28 ]. No consistent trends were observed in IL-5, IgE, CCL17, CCL26, ILC2s, or circulating basophils in patients with CRSwNP, or IgE, CCL17, and hsCRP in patients with AD. This may indicate that the type 2 inflammatory response may not have been fully inhibited by PF-06817024 either due to insufficient IL-33 neutralization or by the involvement of alternative pathways inducing type 2 inflammation. This could include other alarmins such as IL-25 that also promote the production of cytokines such as IL-4, IL-5, and IL-13, but are not inhibited by PF-06817024 [ 29 , 30 ]. Considering the role of IL-33 and the alarmin thymic stromal lymphopoietin in activating the type 2 immune response [ 31 ], a combined blockade of both cytokines may be beneficial in patients with immunological conditions such as asthma or CRSwNP.
Total IL-33 levels were measured in the study as a surrogate of target engagement, and they appeared to have reached their maximum level/plateau by Day 61 in Part 2 (patients with CRSwNP) and Day 113 in Part 3 (patients with AD). These findings further support the a priori selection of these time points for assessment of exploratory efficacy and biomarker endpoints in these patient populations [ 18 ].
PF-06817024 had an effect on clinical outcome measures of CRSwNP severity, including improvement in symptoms of rhinosinusitis (USPIT and SNOT-22 scores), reduction in nasal polyp size and degree of nasal obstruction, and, in patients with comorbid asthma, improved asthma control (ACQ-5).
PF-06817024 demonstrated efficacy in patients with AD, as indicated by mean reductions in disease severity (EASI scores, IGA, BSA, and SCORAD) and patient-reported symptoms (pruritus and ACQ-5). Interestingly, the effects on the mean percentage change from baseline in EASI appeared to be reflective of dichotomous responses in two populations of patients: a responder population with high levels of improvement and a non-responder population who experienced placebo-like effects following administration of PF-06817024. No baseline characteristics could be identified that differentiated responders from non-responders (data not shown), which makes it difficult to predict who will benefit most from treatment with PF-06817024 in patients with AD. The modest aggregate clinical effects seen in the current study with PF-06817024 are consistent with results from clinical trials with other IL-33 inhibitors such as itepekimab and etokimab; however, these trials were conducted in patients with asthma and AD, respectively [ 28 , 32 ]. It is not clear if similar bimodal responses have been seen with these other investigational products.
The assessments of PD and signs of clinical efficacy in patients with CRSwNP and AD described herein were done in the context of a dose-escalation, Phase 1 study to assess the safety, tolerability, PK, and immunogenicity of PF-06817024, principally conducted in healthy participants. The exploratory data with PF-06817024 in patients with CRSwNP and AD were generated to evaluate early in its development the potential of PF-06817024 as a treatment for allergic diseases. The study was not designed nor powered for clinical efficacy as would be appropriate for Phase 2 studies. Nevertheless, exploring the activity of PF-06817024 in small cohorts enabled an efficient preliminary assessment of PD and clinical activity to inform future clinical development efforts. It remains to be determined whether prolonged treatment with PF-06817024 would be beneficial in patients with CRSwNP and AD.
In conclusion, PF-06817024 demonstrated modest clinical efficacy in reducing signs and symptoms of both CRSwNP and AD. Further investigation in appropriately powered Phase 2 studies would be necessary to define more fully the potential therapeutic benefit of PF-06817024 in CRSwNP and AD.
Data availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Abbreviations
5-item version of the Asthma Control Questionnaire
atopic dermatitis
analysis of covariance
body mass index
body surface area
CC motif chemokine ligand 17
CC motif chemokine ligand 26
confidence interval
chronic rhinosinusitis with nasal polyps
computerized axial tomography
Eczema Area and Severity Index
≥ 50% improvement from baseline in Eczema Area and Severity Index
≥ 75% improvement from baseline in Eczema Area and Severity Index
≥ 90% improvement from baseline in Eczema Area and Severity Index
forced expiratory volume in 1 s
Good Publication Practice
high-sensitivity C-reactive protein
investigator global assessment
immunoglobulin E
interleukin
type 2 innate lymphoid cells
intravenous
least squares mean
mixed model for repeated measures
nasal polyp score
pharmacodynamics
pharmacokinetics
patient-reported outcome
Scoring Atopic Dermatitis
standard deviation
22-item Sino-nasal Outcome Test
suppression of tumorigenicity 2
University of Pennsylvania Smell Identification Test
Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. 2014;7(1):12.
Article PubMed PubMed Central Google Scholar
Sweeney A, Sampath V, Nadeau KC. Early intervention of atopic dermatitis as a preventive strategy for progression of food allergy. Allergy Asthma Clin Immunol. 2021;17(1):30.
Chen S, Zhou A, Emmanuel B, Thomas K, Guiang H. Systematic literature review of the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyposis. Curr Med Res Opin. 2020;36(11):1897–911.
Article CAS PubMed Google Scholar
Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol. 2015;136(6):1442–53.
Baiardini I, Paoletti G, Mariani A, Malvezzi L, Pirola F, Spriano G, et al. Nasal polyposis quality of life (NPQ): development and validation of the first specific quality of life questionnaire for chronic rhinosinusitis with nasal polyps. Healthc (Basel). 2022;10(2):253.
Google Scholar
Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–22.
Article PubMed Google Scholar
Silverberg JI, Barbarot S, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126(4):417–28. e2.
Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–93.
Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):52.
Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Symptoms and diagnosis of anxiety and depression in atopic dermatitis in U.S. adults. Br J Dermatol. 2019;181(3):554–65.
Article CAS PubMed PubMed Central Google Scholar
Li JC, Fishbein A, Singam V, Patel KR, Zee PC, Attarian H, et al. Sleep disturbance and sleep-related impairment in adults with atopic dermatitis: a cross-sectional study. Dermatitis. 2018;29(5):270–7.
Akdis CA, Arkwright PD, Bruggen MC, Busse W, Gadina M, Guttman-Yassky E, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–605.
Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3(10):e3331.
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90.
Vocca L, Di Sano C, Uasuf CG, Sala A, Riccobono L, Gangemi S, et al. IL-33/ST2 axis controls Th2/IL-31 and Th17 immune response in allergic airway diseases. Immunobiology. 2015;220(8):954–63.
Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130(6):1344–54.
Zhang L, Jiang LL, Cao ZW. Interleukin-33 promotes the inflammatory reaction in chronic rhinosinusitis with nasal polyps by NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21(20):4501–8.
CAS PubMed Google Scholar
Danto SI, Tsamandouras N, Reddy P, Gilbert S, Mancuso J, Page K, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of PF-06817024 in healthy participants, participants with chronic rhinosinusitis with nasal polyps, and participants with atopic dermatitis: a phase 1, randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 2024;64(5):529–43.
Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–4.
Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol. 2006;118(5 Suppl):S17–61.
Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2 Pt 1):176–8.
Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–54.
Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–7.
Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–7.
Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181(9):5981–9.
Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–50.
Angulo EL, McKernan EM, Fichtinger PS, Mathur SK. Comparison of IL-33 and IL-5 family mediated activation of human eosinophils. PLoS ONE. 2019;14(9):e0217807.
Kosloski MP, Kalliolias GD, Xu CR, Harel S, Lai CH, Zheng W, et al. Pharmacokinetics and pharmacodynamics of itepekimab in healthy adults and patients with asthma: phase I first-in-human and first-in-patient trials. Clin Transl Sci. 2022;15(2):384–95.
Salter BM, Oliveria JP, Nusca G, Smith SG, Tworek D, Mitchell PD, et al. IL-25 and IL-33 induce type 2 inflammation in basophils from subjects with allergic asthma. Respir Res. 2016;17:5.
Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15(6):985–95.
Stanbery AG, Shuchi S, von Jakob M, Tait Wojno ED, Ziegler SF, TSLP. IL-33, and IL-25: not just for allergy and helminth infection. J Allergy Clin Immunol. 2022;150(6):1302–13.
Chen YL, Gutowska-Owsiak D, Hardman CS, Westmoreland M, MacKenzie T, Cifuentes L, et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci Transl Med. 2019;11(515):eaax2945.
Download references
Acknowledgements
The authors would like to thank all the participants, investigators, and study site personnel involved in this study. They would also like to thank Maria Kudela for her contribution as study statistician. Medical writing support, under the direction of the authors, was provided by Megan Melody, MSc, CMC Connect, a division of IPG Health Medical Communications and was funded by Pfizer, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022;175:1298–1304).
This study was sponsored by Pfizer.
Author information
Jean S. Beebe: Affiliation at the time the study was conducted.
Authors and Affiliations
Pfizer Inc, 1 Portland Street, Cambridge, MA, 02151, USA
Spencer I. Danto, Nikolaos Tsamandouras, Padma Reddy, Steven A. Gilbert, Jessica Y. Mancuso, Karen Page, Jean S. Beebe, Elena Peeva & Michael S. Vincent
You can also search for this author in PubMed Google Scholar
Contributions
SID: Conceptualization, and writing – review and editing. NT: Conceptualization, and writing – review and editing. PR: Conceptualization (Part 3), study conduct (Parts 2 and 3), writing – review and editing. SAG: Conceptualization, formal analysis, methodology, and writing – review and editing. JYM: Data analysis, interpretation, and writing – review and editing. KP: Conceptualization, methodology, visualization, and writing – review and editing. JSB: Conceptualization, writing – review and editing. EP: writing – review and editing. MSV: writing – review and editing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Spencer I. Danto .
Ethics declarations
Ethics approval and consent to participate.
The study was conducted in compliance with the ethical principles derived from the Declaration of Helsinki and all International Council for Harmonisation Good Clinical Practice guidelines. The final protocol, any amendments, and informed consent documentations were approved by the Western Institutional Review Board and the UC Davis Office of Research: Institutional Review Board. Written informed consent to participate in the study was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
All authors are employees of and hold stock in Pfizer Inc.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Danto, S.I., Tsamandouras, N., Reddy, P. et al. Exploratory pharmacodynamics and efficacy of PF-06817024 in a Phase 1 study of patients with chronic rhinosinusitis and atopic dermatitis. Allergy Asthma Clin Immunol 20 , 46 (2024). https://doi.org/10.1186/s13223-024-00894-8
Download citation
Received : 19 December 2023
Accepted : 23 April 2024
Published : 30 August 2024
DOI : https://doi.org/10.1186/s13223-024-00894-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anti-IL-33 antibody
- Chronic rhinosinusitis with nasal polyps
- Atopic dermatitis
- Pharmacodynamics
Allergy, Asthma & Clinical Immunology
ISSN: 1710-1492
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Asthma Allergy
- PMC11348984
A Fully Decentralized Randomized Controlled Study of As-Needed Albuterol–Budesonide Fixed-Dose Inhaler in Mild Asthma: The BATURA Study Design
Craig laforce.
1 North Carolina Clinical Research, Chapel Hill, NC, USA
Frank C Albers
2 Avillion, Northbrook, IL, USA
Mark Cooper
3 BioPharmaceuticals Research and Development, AstraZeneca, Cambridge, UK
Anna Danilewicz
4 Avillion, London, UK
Lynn Dunsire
Robert rees, christy cappelletti.
5 BioPharmaceuticals Research and Development, AstraZeneca, Durham, NC, USA
Associated Data
The datasets used and analyzed during the study described in this manuscript may be obtained in accordance with the data sharing policy of Avillion’s co-development partner AstraZeneca, as described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure .
Decentralized clinical trials, where trial-related activities occur at locations other than traditional clinical sites(eg participant homes, local healthcare facilities), have the potential to improve trial access for people for whom time and/or distance constraints may impede participation. Albuterol–budesonide 180/160 µg pressurized metered-dose inhaler (pMDI) is FDA approved for the as-needed treatment or prevention of bronchoconstriction and to reduce the risk of exacerbations in patients with asthma 18 years or older. BATURA (NCT05505734) is a fully decentralized study, investigating as-needed albuterol–budesonide in participants with mild asthma.
BATURA is a fully decentralized, phase 3b, randomized, double-blind, event-driven exacerbation study conducted in the United States. Participants aged ≥12 years using as-needed short-acting β 2 -agonist (SABA), alone or with low-dose inhaled corticosteroid or leukotriene receptor antagonist maintenance, are randomized 1:1 to as-needed albuterol–budesonide 180/160 µg or albuterol 180 µg pMDI for up to 52 weeks (minimum 12 weeks). Participants continue their current maintenance therapy, if applicable. Participants must have used SABA for ≥2 days in the 2 weeks pre-enrollment and have an Asthma Impairment Risk Questionnaire score ≥2 at screening and randomization. All trial-related visits, including screening and consent, are conducted virtually, with study medication shipped directly to each participant’s residence. The primary objective is to evaluate the efficacy of as-needed albuterol–budesonide versus albuterol on severe asthma exacerbation risk, measured by time-to-first severe asthma exacerbation (primary endpoint). Secondary endpoints include annualized rate of severe asthma exacerbation and total systemic corticosteroid exposure. Study medication use is captured via a Hailie sensor attached to the study medication pMDI. The intended sample size is 2500 participants.
BATURA evaluates as-needed albuterol–budesonide in participants with mild asthma. The decentralized study model enables the trial to move out of research sites into participant homes, reducing participant burden and improving access.
Introduction
Clinical trials have traditionally been conducted at specific clinical research sites. However, advances in digital health technologies, including telemedicine, digital healthcare apps, digital imaging, and internet-connected remote sensors, increasingly support accurate, precise, remote data collection. 1–6 These technical innovations, alongside changes in clinical trial implementation strategies necessitated by the COVID-19 pandemic, have catalyzed the adoption of decentralized clinical trial models. 3 , 6 , 7 Decentralized trials are those where some (or all) of the activities related to a clinical trial occur at locations other than traditional clinical research sites, including participant homes, local healthcare facilities, or nearby laboratories. 8 Fully decentralized trials, where all trial-related activities occur in non-clinical settings (eg participant homes), 2 , 5 , 9 present several potential advantages over research site-based clinical trials, including improved trial access for participants who might not otherwise have the opportunity to participate due to time/distance constraints, thus facilitating diversity in the populations recruited. 2 , 4 , 10 Furthermore, these trials have the potential to observe near-real-world participant behaviors, clinical events, and responses, 1 , 3 whilst reducing the burden of participation. 2 , 11
Decentralized trial designs are particularly suitable for studying conditions that can be managed using a telemedical approach, especially when endpoint measurements do not require participants to attend a clinical trial site and the therapies being investigated are self-administered and have well-characterized safety profiles. 5 Asthma is usually managed on an outpatient basis, using self-administered therapies, 12 , 13 therefore making trials investigating this chronic disease suitable for decentralization. As an increasing number of trials are being conducted in a decentralized manner, regulators worldwide, including the United States Food and Drug Administration (FDA) and the European Medicines Agency, have issued recommendations for implementing the decentralization of clinical trials in a participant-centric and risk-proportionate manner. 5 , 14
Asthma exacerbations can have a significant impact on the lives of people with asthma, representing a major health burden. Even patients considered to have mild asthma are at risk of acute deteriorations of their asthma symptoms that can lead to severe exacerbations. Indeed, a recent analysis of real-world data (2010–2017) from patients with mild asthma in the United States found that 57% of patients being treated with as-needed short-acting β 2 -agonist (SABA) only, and 42% treated with low-dose inhaled corticosteroid (ICS) or leukotriene receptor antagonist (LTRA) maintenance (plus as-needed SABA), experienced ≥1 severe exacerbation requiring systemic corticosteroids annually. 15 As airway inflammation is central to asthma symptoms and exacerbations, current expert international asthma treatment reports and guidelines (including the Global Initiative for Asthma [GINA] report and the National Asthma Education and Prevention Program [NAEPP] guidelines) advocate the concomitant treatment of symptoms and inflammation. 12 , 13 However, during periods when asthma symptoms worsen, patients typically reach for SABA rescue inhalers, which treat symptoms, but do not address the corresponding increasing inflammation. 16 , 17
Albuterol–budesonide 180/160 µg (AIRSUPRA TM ) is a rescue therapy for asthma that combines a SABA and an ICS in a single pressurized metered-dose rescue inhaler (pMDI). 18 , 19 The MANDALA study showed that this combination can significantly reduce the risk of a severe exacerbation by 28%, compared with as-needed use of albuterol 180 µg, among people aged ≥18 years. 20 Based on these data, and those from the DENALI study, 19 albuterol–budesonide 180/160 µg pMDI was approved by the FDA in 2023 for the as-needed treatment or prevention of bronchoconstriction and to reduce the risk of exacerbations in patients with asthma 18 years of age and older. Although indicated in the United States across all asthma severities, data for as-needed use of albuterol–budesonide 180/160 µg on the reduction of exacerbation risk are limited to people with moderate-to-severe asthma. 18 , 19 Furthermore, while adolescents (12–17 years) were included in MANDALA, data in this age group were inconclusive due to low sample size (n=100) and corresponding low event rates.
Here, we report the design of BATURA (NCT05505734), an ongoing, fully decentralized clinical trial evaluating the efficacy and safety of as-needed albuterol–budesonide 180/160 µg pMDI for rescue in participants ≥12 years old with mild asthma. BATURA is the first study of an inhaled asthma therapy to employ a fully decentralized approach and aligns with FDA guidelines for implementing decentralized clinical trials. BATURA will provide data on exacerbation risk and the safety of albuterol–budesonide 180/160 µg, compared with albuterol 180 µg, in participants with mild asthma in a home-based setting.
Study Design and Procedures
Study overview and objectives.
BATURA is a phase 3b, participant-centric, fully decentralized, randomized, double-blind, parallel-group, event-driven exacerbation study being conducted in the United States. The objective of BATURA is to evaluate the efficacy and safety of albuterol–budesonide 180/160 µg pMDI, compared with albuterol 180 µg pMDI, both used as needed, in participants ≥12 years old with mild asthma ( Figure 1 ). All trial-related activities, including recruitment, enrollment, informed consent, study visits, and data collection ( Figure 2 ) are conducted virtually.

Overview of trial design.

Bringing the study from the research site to the participant’s home: decentralized components of BATURA.
Patient feedback on the study design was sought via interviews with nine individuals with mild or moderate asthma. Patients were enthusiastic about the decentralized study design with potential improvements in trial convenience and accessibility, and provided guidance on participant-friendly terminology to describe visits (eg “virtual” rather than “remote” or “telemedicine”), which was implemented in all participant-facing materials. Areas of potential concern included data privacy, access to study-related information, and replacement of their current inhalers with study medication. These concerns were addressed by providing assurance on data privacy within the informed consent form, an on-demand patient concierge service (with a team of “patient navigators”) to support both participants and site staff with any study-related questions, and by clarifying, in participant information, that the study inhaler contains similar medication to their current inhalers (albuterol), or similar medication combined with an additional medication (budesonide).
Participants and Eligibility Criteria
A multi-channel approach to recruitment has been implemented, with a significant emphasis on multimedia, multi-platform outreach, including social media, and employing artificial intelligence (AI) technology to rapidly identify high-probability, eligibility-matched participants. The majority of potential participants identified via multimedia outreach are screened remotely for eligibility via the Splash Clinical website ( Figure 2 ). People aged ≥12 years, with a diagnosis of asthma, using as-needed SABA, either alone or with low-dose ICS or LTRA maintenance, are recruited. To be eligible for participation, they must have used SABA on ≥2 days in the 2 weeks pre-enrollment and have an Asthma Impairment and Risk Questionnaire (AIRQ) score ≥2 at screening and randomization (indicating uncontrolled disease). AIRQ is a validated 10-item tool that assesses both the symptom impairment and exacerbation risk domains of asthma control in patients aged ≥12 years. 21–24 Patients with a score of 0–1 are categorized as well controlled, 2–4 as not well controlled, and 5–10 as very poorly controlled. 21 AIRQ control level has been shown to be highly predictive of exacerbation risk over 12 months and probability of time-to-first exacerbation. 22 , 25 , 26
Participants are also required to have access to a smartphone and an internet connection. Other key inclusion and exclusion criteria are presented in Table 1 .
Key Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
Notes : a SABA alone: ≥2 filled prescriptions for a SABA inhaler; SABA + low-dose ICS monotherapy: ≥1 filled prescription for a SABA inhaler and ≥1 filled prescription for low-dose ICS; SABA + LTRA: ≥1 filled prescription for a SABA inhaler and ≥1 filled prescription for a LTRA.
Abbreviations : AIRQ, Asthma Impairment and Risk Questionnaire; ICS, inhaled corticosteroid; ICU, intensive care unit; LABA, long-acting β 2 -agonist; LTRA, leukotriene receptor antagonist; SABA, short-acting β 2 -agonist; SCS, systemic corticosteroid; V, visit.
Study Treatment and Allocation
Eligible participants are randomized 1:1 ( Figure 1 ) to receive as-needed albuterol–budesonide 180/160 µg, given as two inhalations of albuterol/budesonide 90/80 µg, or as-needed albuterol 180 µg, given as two inhalations of albuterol 90 µg, all delivered by pMDI. Study-related supplies, including study medication, are shipped from a central depot directly to the residence of each participant via a climate-controlled courier service. Participants must confirm they have received the allocated study medication at the time of delivery. Each participant received a shipment of between four and six study medication kits at the start of the treatment period (depending on when they were enrolled), with each kit including two study medication pMDIs (total of eight-to-twelve pMDIs). The quantity of medication available to each participant is checked during virtual visits; new study medication kits are ordered by investigators and shipped to participants as required. Participants are to use study medication in place of their normal rescue medication, either in response to symptoms or prior to exercise, and should not exceed 12 inhalations/day of study medication. Study medication use is captured via a sensor attachment (Hailie, Adherium Limited) on the participant’s pMDI, which records each inhaler actuation, but is not built to recognize inhalation technique. Participants are trained, remotely, in the administration, handling, and cleaning of their study medication. All participants prescribed permitted background maintenance therapies (see Table 1 ) continue these medications as instructed by their healthcare provider. Participants are not permitted to use a spacer with study medication pMDIs, but may with their usual maintenance inhalers.
Randomization is assigned using a central randomization and trial supply management system, stratified by each participant’s background therapy and number of severe exacerbations in the 12 months prior to screening. BATURA will continue until either 1) 345 first severe asthma exacerbations have been observed and all patients have completed 12 weeks of study, 2) all participants have completed 52 weeks of treatment, or 3) an unblinded interim analysis (planned to occur once 172 first severe exacerbations have been observed) has determined that the study may be stopped early for demonstrated efficacy.
Digital Health Technologies to Support Decentralization
The SCIENCE37 mobile software application and associated websites are being used to provide digital support to participants and investigators from screening through to study completion ( Figure 2 ). This platform supports electronic informed consent, electronic patient-reported outcome (PRO) questionnaire completion, telemedicine visits, participant education, and eSource documentation. SCIENCE37 also hosts instructional videos and other information helpful to trial participants and investigators, and enables participants to request additional study medication in a blinded fashion. Additionally, the system prompts participants, via bi-weekly notifications, to inform investigators of worsening asthma symptoms ( Figure 2 ) which will then stimulate unscheduled virtual visits to gather further information regarding the potential for a severe asthma exacerbation.
Alongside study medication, each participant receives two Hailie sensors, which automatically log pMDI actuations in real time once attached to an inhaler and paired (via Bluetooth) with the Hailie app on the participant’s smartphone. This allows detailed measurement of the inhaled dose received by each participant, thereby facilitating identification of potential overdose and estimation of ICS exposure resulting from use of study medication. Participants are instructed on how to mount Hailie sensors to their study medication pMDIs and pair with an appropriate device. While the sensors initially provided are expected to have sufficient battery life for the study period and follow-up, additional sensors can be ordered in case of damage or loss. In case of technical difficulties and/or related questions, a telephone-based patient concierge service (Patient Navigator) has been established.
Assessments and Endpoints
Study endpoints are detailed in Figure 3 ; all endpoints were chosen to be safely evaluated without the need for participants to attend a clinical trial site.

Study endpoints.
The primary endpoint is time-to-first severe exacerbation, where an exacerbation is defined as a worsening of asthma signs and/or symptoms and increased use of as-needed rescue medication. Exacerbations are considered severe if they resulted in at least one of the following: a temporary bolus/burst of systemic corticosteroid (SCS) for ≥3 consecutive days (or a single depo-injectable dose of corticosteroids) to treat symptoms of asthma worsening; an emergency room or urgent care visit due to asthma that required SCS (as per the above); inpatient hospitalization due to asthma; or death. The primary analysis is based on an estimand “while-on-treatment” strategy (see Statistical Analysis).
Secondary endpoints include time-to-first severe exacerbation (based on an estimand using an intention-to-treat analysis), annualized rate of severe asthma exacerbations, total SCS exposure (mg/year) per participant, and total days of SCS exposure. Tertiary and exploratory endpoints include healthcare resource use (HCRU), working days lost to asthma-related illness, and PROs measured using the AIRQ and EuroQol-5 Dimension-5 Level (EQ-5D-5L) instrument. Safety endpoints include the number, frequency, severity, type, and outcome of adverse events and serious adverse events.
Statistical Analysis
BATURA is a superiority trial; the primary analysis of time-to-first severe asthma exacerbation will use data collected during the on-treatment period, before treatment discontinuation or a change in maintenance therapy (while-on-treatment strategy). A secondary intention-to-treat analysis will also be conducted for time-to-first severe asthma exacerbation, including all observed data obtained during the study, regardless of participant randomization status, treatment discontinuation, or change in maintenance therapy (treatment policy).
Time-to-first severe asthma exacerbation will be analyzed using a Cox proportional hazards regression model, including factors for treatment, pre-study asthma therapy (SABA only, low-dose ICS + SABA, or LTRA + SABA), and the number of severe exacerbations (0, ≥1) in the 12 months prior to screening. A two-sided test will evaluate the null hypothesis that the adjusted hazard ratio for the primary treatment comparison is equal to 1, versus the alternative hypothesis that it is not equal to 1. Annualized rate of severe asthma exacerbations will be analyzed using a negative binomial generalized linear model, including the same factors as the primary analysis model, with time at risk on the logarithm scale as an offset variable; the annualized rate ratio and 95% confidence interval will be estimated for the treatment comparison. The treatment comparison for annualized SCS exposure will be made using a Wilcoxon rank-sum test, and descriptive statistics will be calculated for adverse event data. The above tests will be type I error controlled via a hierarchical testing procedure, using the following endpoint testing order: time-to-first exacerbation (primary efficacy analysis), time-to-first exacerbation (intention-to-treat efficacy analysis), annualized severe exacerbation rate, and annualized total SCS exposure ( Figure 3 ).
A sample size of 955 participants per treatment group, and a total of 345 first severe asthma exacerbation events, was initially calculated to provide 90.8% power to detect a 30% reduction in the risk of a severe asthma exacerbation for participants using albuterol–budesonide versus albuterol, with a two-sided hypothesis test and overall type I error rate of 5%. This reduction is supported by results from the MANDALA study, in which the risk of a severe exacerbation was reduced by 27% with albuterol–budesonide 180/160 µg, versus albuterol, in participants ≥12 years old with moderate-to-severe asthma. 18 This assumed a first severe exacerbation event probability of 0.21 with the as-needed use of albuterol, based on assessment of real-world studies in participants with mild asthma and an assumed 10% drop-out rate. After a blinded sample size re-estimation, following lower than expected rates of first severe exacerbation, a total trial population of 2500 is being enrolled. An independent data monitoring committee will perform an unblinded interim analysis for efficacy when 172 first severe exacerbation events have been observed, based on analysis of the primary and first secondary endpoints.
Populations with mild asthma are at substantial risk of exacerbations. A recent United States-based healthcare claims study found that 57% of patients with asthma treated according to GINA step 1, and 42% treated according to GINA step 2, had experienced a severe exacerbation in the previous 12 months. 15 In people with asthma, airway inflammation and bronchoconstriction contribute to airway narrowing and airflow limitation, leading to asthma symptoms and exacerbations. 27–29 Both inflammation and symptoms vary over time and in intensity, and can lead to unpredictable exacerbations. 12 , 13 , 28 , 29 People with asthma often reach for rescue therapies, prioritizing quick relief when symptoms occur. However, treatment with SABA-only rescue does not address the corresponding rise in inflammation. Combination rescue therapy with an ICS and a fast-acting bronchodilator can address this issue, since symptoms and inflammation are treated concomitantly when most needed, reducing the risk of an exacerbation. As such, both global and local recommendations on the management of asthma, including the GINA report, recommend combination anti-inflammatory-rescue therapies (containing ICS and either SABA or formoterol) for use across multiple asthma severities. While MANDALA provided evidence of the efficacy of as-needed SABA-ICS on the reduction of exacerbation risk in patients with moderate-to-severe asthma, evidence in mild asthma is limited, and BATURA will fill this data gap.
Consistent with MANDALA, time-to-first severe asthma exacerbation was chosen as the primary endpoint for BATURA, with annualized rate of severe asthma exacerbation and annualized SCS exposure included as secondary endpoints. BATURA also included, as exploratory endpoints, PROs of EQ-5D-5L, 30 to assess participant quality of life, and AIRQ, to assess asthma control. AIRQ is a validated 10-item, low literacy-demand tool that assesses both the symptom impairment and exacerbation risk domains of asthma control in patients aged ≥12 years. 21–24 It comprises seven symptom-based questions with a 2-week recall period and three exacerbation history questions with a 12-month recall period. 21 A longitudinal study of the AIRQ in over 1100 patients with asthma found that worsening baseline control, as indicated by the AIRQ control level, was predictive of increasing exacerbation risk over the subsequent 12 months. 22 This longitudinal study also found that AIRQ rated fewer patients as having well-controlled asthma who had current symptom impairment or previous- and subsequent-year exacerbations compared with the Asthma Control Test, the GINA symptom control tool, or expert specialist opinion. 22 , 26
BATURA is the first fully decentralized trial for asthma, and has been designed to align with the FDA guidance document on Decentralized Clinical Trials for Drugs, Biological Products, and Devices ( Table 2 ). 5 The participant group enrolled is at low risk of harm as they are currently managed in an outpatient setting using self-administered therapies with well-characterized safety profiles. 12 , 13 In addition, no complex medical assessments are required to evaluate the endpoints selected. Furthermore, the fully decentralized design of the BATURA trial allows participants to fit trial activities around their everyday lives, thus lessening participant burden. Data are collected in the home environment, using familiar devices and an automated sensor. 1 , 3 This approach also reduces the burden of travel which, as well as being more convenient to participants, may reduce per-trial carbon footprint. 31 , 32 It is hoped decentralization will encourage a more diverse range of people to be enrolled, therefore improving the representation of groups which have been under-represented in clinical trial populations.
BATURA Study Design Aligns with FDA Guidelines for Use of a Decentralized Approach
| Study Characteristics Suitable for a Decentralized Design | BATURA |
|---|---|
| Therapies are simple to administer or use | Study drug is self-administered via pMDI, the most commonly prescribed medical device worldwide |
| Therapies have well-characterized safety profiles | Albuterol–budesonide: |
| Study does not require complex medical assessments | Exacerbations as a primary endpoint are well defined |
| Telehealth visits, where in-person interaction is required | All visits are virtual, including screening and consent |
| Digital health technologies can be used to transmit data remotely from trial participants | Study medication use is captured electronically |
| Study-related supplies can be distributed directly to participants at their locations | Study drug and related supplies are shipped directly to participants |
Abbreviations : FDA, United States Food and Drug Administration; pMDI, pressurized- metered dose inhaler.
Trial decentralization can present a number of challenges to implementation, including the need to maintain participant and trial site engagement with remote study procedures and documentation requirements, and the need to remotely ensure compliance with these procedures and documentation requirements. 5 , 9 , 35 Decentralization may improve access to clinical trials by removing geographical barriers to participation, but can create digital barriers for some participants, both in recruitment and with use of digital health technologies during the study (ie ability to install and use sensors and digital platforms without the hands-on assistance of investigators and site staff). To address this concern, participants in BATURA were allowed to use their own Smartphone device and every effort was made to ensure that the study software applications (SCIENCE37 and Hailie portal) were easy to use, with a patient concierge service established to provide technical assistance as necessary. Another challenge of fully decentralized trials is that, by moving the study out of the research site and into the participant’s home, much of the face-to-face interaction involved in site-based clinical trials is removed, which can result in participants having a more distant relationship with both study staff and the study itself, potentially creating challenges to participant engagement and retention. Despite these concerns, the majority of literature concerning patient retention in clinical trials has found that decentralized clinical trials typically have superior rates of retention to those seen in site-based clinical trials. 11 , 36 A decentralized design may also necessitate the exclusion of certain tests and procedures that may have been considered in a site-based study. For example, in BATURA the decision was made to reduce participant burden by not including eDiaries, and lung function assessment was considered to be unfeasible due to the challenges of performing spirometry in a home setting. In general, both technologies and social attitudes are evolving quickly to support studies of this nature; as trial decentralization and digitization become more common, it is likely that improved applications and increased participant and investigator experience will address many such concerns.
The BATURA study, using an innovative decentralized approach, is investigating the efficacy of as-needed albuterol–budesonide 180/160 µg to reduce the risk of severe exacerbations in people with mild asthma, building on what has already been shown in the MANDALA study in patients with moderate-to-severe asthma. 18 , 20
BATURA employs a novel, decentralized study design to evaluate the efficacy and safety of as-needed albuterol–budesonide 180/160 μg in participants ≥12 years of age with mild asthma. This decentralized design enables the study to move out of the clinical research site and into participant homes, thus reducing participant burden, potentially reaching people for whom time and/or distance constraints may impede clinical trial participation, therefore expanding clinical trial access to a broader population.
Acknowledgments
The authors would like to thank Lucy C. Cooper of inScience Communications, Springer Healthcare, UK, for providing medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice 2022 (GPP2022) guidelines ( https://www.ismpp.org/gpp-2022 ).
An abstract of this paper was presented at the CHEST 2023 Annual Meeting as a poster presentation. The poster’s abstract was published in “Poster Abstracts” in Chest 2023; 164 : A2–3: https://doi.org/10.1016/j.chest.2023.07.072 .
Funding Statement
This study is funded by Bond Avillion 2 Development LP. Medical writing support for this manuscript was funded by AstraZeneca.
Abbreviations
AI, artificial intelligence; AIRQ, Asthma Impairment and Risk Questionnaire; eICF, electronic informed consent form; EQ-5D-5L, EuroQol-5 Dimension-5 Level; FDA, United States Food and Drug Administration; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroid; ICU, intensive care unit; LABA, long-acting β 2 -agonist; LTRA, leukotriene receptor antagonist; pMDI, pressurized metered-dose inhaler; R, randomization; SABA, short-acting β 2 -agonist; SCS, systemic corticosteroid.
Data Sharing Statement
Ethics approval and consent to participate.
BATURA is being performed in accordance with the Declaration of Helsinki and is consistent with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and Good Clinical Practice (ICH/GCP) and applicable regulatory requirements. All portions of the protocol, electronic informed consent form (eICFs), and other relevant documents have been approved by an institutional review board (ADVARRA, Maryland, US).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
CL has received industry-initiated research funding (eg clinical trials) in the past 24 months from Amphastar, AstraZeneca, Avillion, Chiesi, Chugai Pharmaceutical, Cipla, DawnLight, Eli Lilly & Co, GlaxoSmithKline, Kanyos Bio, Knopp Biosciences, Mako Medical Labs, Novartis, Satsuma Pharmaceuticals, Teva, Theravance, UCB Biopharma, and Vorso, and acted as a consultant for AstraZeneca. FCA, AD , and RR are employees of Avillion. CC, MC , and LD are employees of and hold stock in AstraZeneca. The authors report no other conflicts of interest in this work.

IMAGES
VIDEO
COMMENTS
The first large-scale multicenter randomized controlled study was the Asthma Intervention Research (AIR) Trial, which enrolled patients with moderate to severe asthma. 10 In this trial, patients ...
Severe/Critical asthma is a life threatening condition. Asthma Pathophysiology. Asthma is a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role, in particular, mast cells, eosinophils, T lymphocytes, macrophages, neutrophils, and epithelial cells. The inflammation in asthma cause recurrent episode ...
A 66-year-old man, an asthmatic, presented with symptoms suggestive of an acute exacerbation of asthma. His arterial blood gas revealed type 1 respiratory failure (PaO 2 <8 kPa or 60 mm Hg with normal or low PaCO 2) with a compensated lactic acidosis. He was treated for an asthma exacerbation and sepsis. Despite treatment, his respiratory rate ...
Despite the theoretical benefits of heliox, and while a few case series have suggested a beneficial effect in acute asthma, no studies in adults have demonstrated an advantage of heliox above and beyond standard oxygen therapy. In asthma exacerbation either without or with intubation, heliox has not demonstrated consistent benefit [247,248].
Current studies show that Sevoflurane has significant bronchodilator properties and is an effective treatment option for severe acute asthma before rescue therapies [9]. The beneficial effect of Sevoflurane in our case is supported by alveolar unit and distal airway dilation, which reduce distortion of the surrounding parenchyma and amount of ...
Once the acute exacerbation has been managed, most patients will be well enough for discharge from the emergency department. In both Canada and the United States, however, relapse rates remain unacceptably high, ranging from 12% to 16% within two weeks after treatment in the emergency department in one recent study. 17 To reduce the risk of relapse, optimizing management after discharge should ...
A 20-year-old woman with a history of severe asthma was found at home in an unresponsive state and was taken to the emergency room. Asthma had been diagnosed at 4 years of age, with multiple exacer...
Dr. Lila M. Martin: A 73-year-old man was transferred to the intensive care unit (ICU) of an academic health center in Boston for acute hypoxemic respiratory failure in March 2020, during the ...
The prevalence of asthma in adults in the United States is approximately 7.7%. 1 It is one of the most common chronic, noncommunicable diseases in the country and worldwide. 1,2 Among U.S. adults ...
Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases (p < 0.001), while FEV 1 improved by 4.9% (p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% (p < 0.001) .
In one study, of 33,000 patients with acute asthma exacerbation requiring hospital care, 10.1% required admission to the ICU and 2.1% required intubation and invasive mechanical ventilation (IMV). 3 Therefore, it is imperative for clinicians working in an ICU to be familiar with the proper assessment and management strategies of life ...
A number of studies have demonstrated the challenges for primary care physicians in providing ongoing support for people with asthma. 31,48,49 In some countries, nurses and other allied health ...
Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation Lopamudra Das, MD, Lopamudra Das, MD ... Population-based studies have shown that 20% to 35% of children with allergies experience bullying. In many cases (31% in one recent study ), this bullying is related directly to the food allergy. From a medical perspective ...
Despite, Acute asthma attack is an important public health problem that affects not only the patients, but also to the family, health professionals, health care institutions and development of the nation, little is known about the risk factors of acute asthma attack. Therefore, this study is aimed to investigate the determinants of acute asthma ...
Primary Objective. Objective: RS4.4: Asthma. Compare and contrast the clinicopathological features and causes of asthma and describe the morphologic changes and consequences that result in airflow obstruction. Competency 2: Organ System Pathology; Topic: Respiratory System (RS); Learning Goal 4: Obstructive Diseases of the Lung.
CASE PRESENTATION: A 58 years old Caucasian male, non smoker, with late onset allergic asthma was referred to our pulmonary rehabilitation clinic because of deconditioning, wheezing and recurrent asthma exacerbations despite treatment with budesonide-formoterol 200/6 mcg b.i.d., montelukast 10 mg q.i.d. He had daily complaints of dyspnea both at rest and on exertion using almost 200 ...
Pneumonia associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and acute pulmonary embolism. Notes This case was presented at the Medicine Case Conference.
Regular use of inhaled steroids reduces severe exacerbations of asthma 23 and the need for bronchodilators, 24 while the prompt use of systemic corticosteroids during an exacerbation reduces the need for hospital admissions, use of β agonists, 25 and relapses. 26. The evidence for corticosteroid use in early covid-19 is still emerging.
This is especially important for patients with moderate to severe asthma. According to a 2001 study, asthma action plans are associated with a 70% reduction in the risk of death. 13 Asthma action ...
To promote comfort. - Monitor VS capacity without 6. Educate on energy 6. To prevent over- - Assess difficulty or conservation techniques. exertion and relapse. motor fatigue for 24 7. Limit visitors as function hours needed. 7. To reduce stimuli. - Note - Participate 8. Administer medications 8.
Although previous studies among patients with asthma or rheumatic disease have suggested associations between long-term use of oral corticosteroids and various adverse events (AEs), there are few studies of patients with AD, to our knowledge. 15-21 In addition, existing studies about corticosteroid use among patients with AD were conducted to ...
CASE REPORT. In January 2020, a 53-year-old gentleman with a background of asthma on long-term low dose inhaled corticosteroid inhaler had an acute exacerbation of his asthma in February 2020 triggered by a viral upper respiratory tract infection and acute sinusitis and was managed with bronchodilator nebulization and a 7-day course of oral prednisone 30 mg daily.
Uncontrolled asthma is associated with an increased risk of adverse perinatal outcomes. Asthma biologics reduce exacerbation frequency, are steroid sparing, and improve quality of life in people with severe asthma. However, evidence for the use and safety of asthma biologics during pregnancy is scarce, largely because pregnant women were excluded from clinical trials. To help to support ...
A randomized crossover study that compared the use of bilevel positive airway pressure for 2 hours with standard care in children with acute asthma showed a significantly lower respiratory rate ...
In severe cases, these symptoms may pose life-threatening risks. 6 The present case report discusses a patient who inadvertently inhaled diesel oil during a diesel engine repair. The presenting symptoms of cough, scant sputum, respiratory discomfort, chest tightness, and dyspnea were indicative of acute respiratory distress, aligning with ...
A 48-year-old female patient with uncontrolled severe asthma was referred to our hospital for anti-IgE therapy. She was suffering with persistent wheezing and dyspnea after a severe asthma attack that had taken place 5 months previously. Her asthma had not been controlled with adequate asthma treatment, including budesonide at 320 μg ...
PF-06817024 is a humanized antibody against interleukin-33 that has the potential to inhibit type 2 inflammation. An exploratory analysis of the pharmacodynamics and clinical effects of single and repeat doses of PF-06817024 was assessed in patients with chronic rhinosinusitis with nasal polyps (CRSwNP) and patients with moderate-to-severe atopic dermatitis (AD), respectively, as part of a ...
A recent United States-based healthcare claims study found that 57% of patients with asthma treated according to GINA step 1, and 42% treated according to GINA step 2, had experienced a severe exacerbation in the previous 12 months. 15 In people with asthma, airway inflammation and bronchoconstriction contribute to airway narrowing and airflow ...