Breakthrough diabetes study could lead to end of regular insulin injections, researchers say
Researchers say they have made a breakthrough in the treatment of type 1 diabetes which could replace the need for regular insulin injections.
Research published by Baker Heart and Diabetes Institute scientists shows they have manipulated existing pancreatic stem cells to prompt them to produce insulin.
The study from the Melbourne researchers builds on previous work by Monash University scientists, using two existing cancer drugs.
The research is still in its early days and the next step will be pre-clinical animal trials.
But lead researcher and Baker institute scientist, Sam El-Osta, said the potential treatment could be viable for children and adults in the future.
"What we've discovered is the ability to harness the patient's remaining pancreatic cells to influence those cells to behave like insulin-producing beta cells," Professor El-Osta told ABC Radio Melbourne.
"This could potentially modify the course of diabetes and potentially eliminate the need for round-the-clock insulin injections in some people living with type 1 diabetes."
Broadly, people with diabetes do not naturally produce enough insulin, or their bodies do not use the hormone as they should.
For many people with diabetes, it means multiple insulin injections are required daily to manage the illness.

Research could be 'holy grail'
The two cancer drugs used in the research are already approved by the US Food and Drug Administration.
Researchers said the potential treatment could be "rapid" compared to current treatment options for type 1 diabetes.
"We've been able to repurpose these drugs to determine whether we could influence the trajectory by using these small molecule inhibitors in pancreatic ductal cells," Professor El-Osta said.
"We can quickly influence insulin restoration in a number of days in a dish from tissues derived from type 1 diabetes donors, both children and adults."
Diabetes Australia estimates around 134,000 people in Australia are living with type 1 diabetes, which represents about 10 per cent of all diabetes cases.
The Baker Heart and Diabetes Institute researchers are optimistic their work could potentially help people living with insulin-dependent type 2 diabetes.
The research has been published in a Nature scientific journal, Signal Transduction and Targeted Therapy.
Chief executive of the Australian Diabetes Society and University of Melbourne associate professor, Sof Andrikopoulos, labelled the research as "remarkable".
"For the 135,000 Australians with type 1 diabetes, this is the holy grail. This is it," he said.
"There's a potential here that this research might lead to the cure of type 1 diabetes, at some point down the road.
"It also has the potential to make a significant improvement in type 2 diabetes."
Dr Andrikopoulos, who was not involved with the study, said the research would reduce the burden of the disease.
"This research has the potential for the body itself, to produce and secrete insulin. So you can see that you're getting rid of needles, you're getting rid of insulin pumps, you're getting rid of finger pricking, you're getting rid of continuous glucose monitors," he said.
While he was hopeful for the future, Dr Andrikopoulos warned steps towards a cure would require consistent funding for diabetes research.
- X (formerly Twitter)
- Science and Technology

- August 5, 2024 | Head Injuries and Heartache: The Police Story You Haven’t Heard
- August 5, 2024 | Unlocking Future Technologies With Magnetic Control of Rare Earth Elements
- August 5, 2024 | Thought To Be Exclusive to Humans: Scientists Uncover Remarkable Cognitive Skills of Fruit Bats
- August 5, 2024 | The Paradox of Cognition: Why Thinking Too Hard Can Make You Miserable
- August 5, 2024 | When Heart Transplants for Kids Go Wrong: Stanford’s Startling Discovery
Beyond Blood Sugar Control: New Target for Curing Diabetes Unveiled
By Helmholtz Munich March 22, 2024
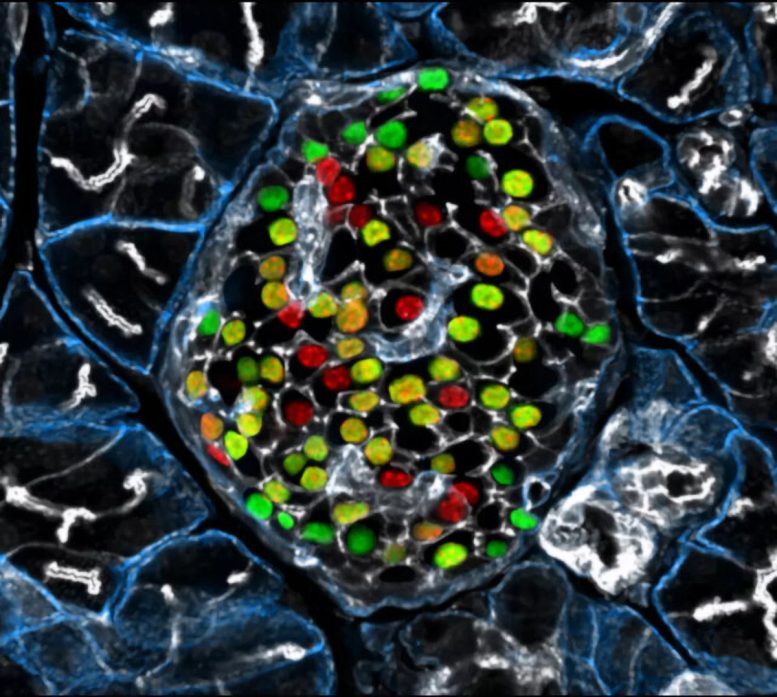
Targeting the inceptor receptor could lead to breakthrough treatments for diabetes by protecting beta cells and improving blood sugar control, with German research institutions leading this promising discovery. Insulin-producing beta cells in the islet of Langerhans. Credit: Helmholtz Munich | Erik Bader
Research focusing on the insulin -inhibitory receptor, known as inceptor, has revealed promising paths for protecting beta cells, providing optimism for therapy that directly addresses diabetes. A groundbreaking study involving mice with obesity caused by diet shows that eliminating inceptor improves glucose management. This finding encourages further investigation into inceptor as a potential therapeutic target for treating type 2 diabetes.
These findings, led by Helmholtz Munich in collaboration with the German Center for Diabetes Research, the Technical University of Munich, and the Ludwig-Maximilians-University Munich, drive advancements in diabetes research.
Targeting Inceptor to Combat Insulin Resistance in Beta Cells
Insulin resistance, often linked to abdominal obesity, presents a significant healthcare dilemma in our era. More importantly, the insulin resistance of beta cells contributes to their dysfunction and the transition from obesity to overt type 2 diabetes. Currently, all pharmacotherapies, including insulin supplementation, focus on managing high blood sugar levels rather than addressing the underlying cause of diabetes: beta cell failure or loss. Therefore, research into beta cell protection and regeneration is crucial and holds promising prospects for addressing the root cause of diabetes, offering potential avenues for causal treatment.
With the recent discovery of inceptor, the research group of beta cell expert Prof. Heiko Lickert has uncovered an interesting molecular target. Upregulated in diabetes, the insulin-inhibitory receptor inceptor may contribute to insulin resistance by acting as a negative regulator of this signaling pathway. Conversely, inhibiting the function of the inceptor could enhance insulin signaling – which in turn is required for overall beta cell function, survival, and compensation upon stress.
In collaboration with Prof. Timo Müller, an expert in molecular pharmacology in obesity and diabetes, the researchers explored the effects of inceptor knock-out in diet-induced obese mice. Their study aimed to determine whether inhibiting inceptor function could also enhance glucose tolerance in diet-induced obesity and insulin resistance, both critical pre-clinical stages in the progression toward diabetes. The results were now published in Nature Metabolism .
Removing Inceptor Improves Blood Sugar Levels in Obese Mice
The researchers delved into the effects of removing inceptor from all body cells in diet-induced obese mice. Interestingly, they found that mice lacking inceptor exhibited improved glucose regulation without experiencing weight loss, which was linked to increased insulin secretion in response to glucose. Next, they investigated the distribution of inceptor in the central nervous system and discovered its widespread presence in neurons. Deleting inceptor from neuronal cells also improved glucose regulation in obese mice. Ultimately, the researchers selectively removed the inceptor from the mice’s beta cells, resulting in enhanced glucose control and a slight increase in beta cell mass.
Research for Inceptor-Blocking Drugs
“Our findings support the idea that enhancing insulin sensitivity through targeting inceptor shows promise as a pharmacological intervention, especially concerning the health and function of beta cells,” says Timo Müller. Unlike intensive early-onset insulin treatments, utilizing inceptor to enhance beta cell function offers promise in alleviating the detrimental effects on blood sugar and metabolism induced by diet-induced obesity. This approach avoids the associated risks of hypoglycemia-associated unawareness and unwanted weight gain typically observed with intensive insulin therapy.
“Since inceptor is expressed on the surface of pancreatic beta cells, it becomes an accessible drug target. Currently, our laboratory is actively researching the potential of several inceptor-blocking drug classes to enhance beta cell health in pre-diabetic and diabetic mice. Looking forward, inceptor emerges as a novel and intriguing molecular target for enhancing beta cell health, not only in prediabetic obese individuals but also in patients diagnosed with type 2 diabetes,” explains Heiko Lickert.
Reference: “Global, neuronal or β cell-specific deletion of inceptor improves glucose homeostasis in male mice with diet-induced obesity” by Gerald Grandl, Gustav Collden, Jin Feng, Sreya Bhattacharya, Felix Klingelhuber, Leopold Schomann, Sara Bilekova, Ansarullah, Weiwei Xu, Fataneh Fathi Far, Monica Tost, Tim Gruber, Aimée Bastidas-Ponce, Qian Zhang, Aaron Novikoff, Arkadiusz Liskiewicz, Daniela Liskiewicz, Cristina Garcia-Caceres, Annette Feuchtinger, Matthias H. Tschöp, Natalie Krahmer, Heiko Lickert and Timo D. Müller, 28 February 2024, Nature Metabolism . DOI: 10.1038/s42255-024-00991-3
More on SciTechDaily

Quantum Breakthrough Reveals Superconductor’s Hidden Nature
Novel Molecules Discovered to Combat Asthma and COVID-Related Lung Diseases
Orbital Engineering, Yale Engineers Change Electron Trajectories

Finding and Erasing Quantum Computing Errors in Real-Time

“Glow-in-the-Dark” Proteins: The Future of Viral Disease Detection?
A black hole – a million times as bright as our sun – offers potential clue to reionization of universe.

Risk Factors for Falls in Older Americans Identified – A Growing Public Health Concern

Common Fireworks Emit Toxic Metals Into the Air – Damage Human Cells and Animal Lungs
1 comment on "beyond blood sugar control: new target for curing diabetes unveiled".
Interesting study and hopefully another tool which will apply to diabetic patients.
Leave a comment Cancel reply
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

If it feels too hot to run, maybe it is
Does your brain reflect your sex?

Beginning of end of HIV epidemic?

“When my son was diagnosed [with Type 1], I knew nothing about diabetes. I changed my research focus, thinking, as any parent would, ‘What am I going to do about this?’” says Douglas Melton.
Kris Snibbe/Harvard Staff Photographer
Breakthrough within reach for diabetes scientist and patients nearest to his heart
Harvard Correspondent
100 years after discovery of insulin, replacement therapy represents ‘a new kind of medicine,’ says Stem Cell Institute co-director Douglas Melton, whose children inspired his research
When Vertex Pharmaceuticals announced last month that its investigational stem-cell-derived replacement therapy was, in conjunction with immunosuppressive therapy, helping the first patient in a Phase 1/2 clinical trial robustly reproduce his or her own fully differentiated pancreatic islet cells, the cells that produce insulin, the news was hailed as a potential breakthrough for the treatment of Type 1 diabetes. For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the challenges ahead, and the personal side of his research. The interview was edited for clarity and length.

Douglas Melton
GAZETTE: What is the significance of the Vertex trial?
MELTON: The first major change in the treatment of Type 1 diabetes was probably the discovery of insulin in 1920. Now it’s 100 years later and if this works, it’s going to change the medical treatment for people with diabetes. Instead of injecting insulin, patients will get cells that will be their own insulin factories. It’s a new kind of medicine.
GAZETTE: Would you walk us through the approach?
MELTON: Nearly two decades ago we had the idea that we could use embryonic stem cells to make functional pancreatic islets for diabetics. When we first started, we had to try to figure out how the islets in a person’s pancreas replenished. Blood, for example, is replenished routinely by a blood stem cell. So, if you go give blood at a blood drive, your body makes more blood. But we showed in mice that that is not true for the pancreatic islets. Once they’re removed or killed, the adult body has no capacity to make new ones.
So the first important “a-ha” moment was to demonstrate that there was no capacity in an adult to make new islets. That moved us to another source of new material: stem cells. The next important thing, after we overcame the political issues surrounding the use of embryonic stem cells, was to ask: Can we direct the differentiation of stem cells and make them become beta cells? That problem took much longer than I expected — I told my wife it would take five years, but it took closer to 15. The project benefited enormously from undergraduates, graduate students, and postdocs. None of them were here for 15 years of course, but they all worked on different steps.
GAZETTE: What role did the Harvard Stem Cell Institute play?
MELTON: This work absolutely could not have been done using conventional support from the National Institutes of Health. First of all, NIH grants came with severe restrictions and secondly, a long-term project like this doesn’t easily map to the initial grant support they give for a one- to three-year project. I am forever grateful and feel fortunate to have been at a private institution where philanthropy, through the HSCI, wasn’t just helpful, it made all the difference.
I am exceptionally grateful as well to former Harvard President Larry Summers and Steve Hyman, director of the Stanley Center for Psychiatric Research at the Broad Institute, who supported the creation of the HSCI, which was formed specifically with the idea to explore the potential of pluripotency stem cells for discovering questions about how development works, how cells are made in our body, and hopefully for finding new treatments or cures for disease. This may be one of the first examples where it’s come to fruition. At the time, the use of embryonic stem cells was quite controversial, and Steve and Larry said that this was precisely the kind of science they wanted to support.
GAZETTE: You were fundamental in starting the Department of Stem Cell and Regenerative Biology. Can you tell us about that?
MELTON: David Scadden and I helped start the department, which lives in two Schools: Harvard Medical School and the Faculty of Arts and Science. This speaks to the unusual formation and intention of the department. I’ve talked a lot about diabetes and islets, but think about all the other tissues and diseases that people suffer from. There are faculty and students in the department working on the heart, nerves, muscle, brain, and other tissues — on all aspects of how the development of a cell and a tissue affects who we are and the course of disease. The department is an exciting one because it’s exploring experimental questions such as: How do you regenerate a limb? The department was founded with the idea that not only should you ask and answer questions about nature, but that one can do so with the intention that the results lead to new treatments for disease. It is a kind of applied biology department.
GAZETTE: This pancreatic islet work was patented by Harvard and then licensed to your biotech company, Semma, which was acquired by Vertex. Can you explain how this reflects your personal connection to the research?
MELTON: Semma is named for my two children, Sam and Emma. Both are now adults, and both have Type 1 diabetes. My son was 6 months old when he was diagnosed. And that’s when I changed my research plan. And my daughter, who’s four years older than my son, became diabetic about 10 years later, when she was 14.
When my son was diagnosed, I knew nothing about diabetes and had been working on how frogs develop. I changed my research focus, thinking, as any parent would, “What am I going to do about this?” Again, I come back to the flexibility of Harvard. Nobody said, “Why are you changing your research plan?”
GAZETTE: What’s next?
MELTON: The stem-cell-derived replacement therapy cells that have been put into this first patient were provided with a class of drugs called immunosuppressants, which depress the patient’s immune system. They have to do this because these cells were not taken from that patient, and so they are not recognized as “self.” Without immunosuppressants, they would be rejected. We want to find a way to make cells by genetic engineering that are not recognized as foreign.
I think this is a solvable problem. Why? When a woman has a baby, that baby has two sets of genes. It has genes from the egg, from the mother, which would be recognized as “self,” but it also has genes from the father, which would be “non-self.” Why does the mother’s body not reject the fetus? If we can figure that out, it will help inform our thinking about what genes to change in our stem cell-derived islets so that they could go into any person. This would be relevant not just to diabetes, but to any cells you wanted to transplant for liver or even heart transplants. It could mean no longer having to worry about immunosuppression.
Share this article
You might like.
Experts who have seen health consequences close-up offer guidelines for summer athletes

Precision medicine is just one field where the answer matters

Scientists cautiously optimistic about trial results of new preventative treatment, prospects for new phase in battle with deadly virus
The way forward for Democrats — and the country
Danielle Allen is more worried about identity politics and gaps in civic education than the power of delegates
17 books to soak up this summer
Harvard Library staff recommendations cover romance, fantasy, sci-fi, mystery, memoir, music, politics, history
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
New Research Sheds Light on Cause of Type 2 Diabetes

St. Petersburg, Fla. – September 12, 2023 – Scientists at Johns Hopkins All Children’s Hospital, along with an international team of researchers, are shedding new light on the causes of Type 2 diabetes. The new research, published in the journal Nature Communications , offers a potential strategy for developing new therapies that could restore dysfunctional pancreatic beta-cells or, perhaps, even prevent Type 2 diabetes from developing.
The new study shows that the beta-cells of Type 2 diabetes patients are deficient in a cell trafficking protein called “phosphatidylinositol transfer protein alpha” (or PITPNA), which can promote the formation of “little packages,” or intracellular granules containing insulin. These structures facilitate processing and maturation of insulin “cargo.” By restoring PITPNA in the Type 2 deficient beta-cells, production of insulin granule is restored and this reverses many of the deficiencies associated with beta-cell failure and Type 2 diabetes.
Researchers say it’s important to understand how specific genes regulate pancreatic beta-cell function, including those that mediate insulin granule production and maturation like PITPNA to provide therapeutic options for people.
Matthew Poy, Ph.D. , an associate professor of Medicine and Biological Chemistry in the Johns Hopkins University School of Medicine and leader of the Johns Hopkins All Children’s team within the Institute for Fundamental Biomedical Research , was lead researcher on the study. He adds that follow-up work is now focused on whether PITPNA can enhance the functionality of stem-cell-derived pancreatic beta-cells. Since stem cell-based therapies are still in their relatively early stages of clinical development, it appears a great deal of the potential of this approach remains untapped. Poy believes that increasing levels of PITPNA in stem cell-derived beta-cells is an approach that could enhance the ability to produce and release mature insulin prior to transplantation in diabetic subjects.
“Our dream is that increasing PITPNA could improve the efficacy and potency of beta-like stem cells,” Poy says. “This is where our research is heading, but we have to discover whether the capacity of these undifferentiated stem cells that can be converted into many different cell types can be optimized — and to what level — to be converted into healthy insulin producing beta-cells. The goal would be to find a cure for type 2 diabetes.”
Read more about this groundbreaking research.
This study was funded through grants from the Johns Hopkins All Children’s Foundation , the National Institute of Health, the Robert A. Welch Foundation, the Helmholtz Gemeinschaft , the European Foundation for the Study of Diabetes, the Swedish Science Council , the NovoNordisk Foundation and the Deutsche Forschungsgemeinschaft . About Johns Hopkins All Children’s Hospital Johns Hopkins All Children’s Hospital in St. Petersburg is a leader in children’s health care, combining a legacy of compassionate care focused solely on children since 1926 with the innovation and experience of one of the world’s leading health care systems. The 259-bed teaching hospital, stands at the forefront of discovery, leading innovative research to cure and prevent childhood diseases while training the next generation of pediatric experts. With a network of Johns Hopkins All Children’s Outpatient Care centers and collaborative care provided by All Children’s Specialty Physicians at regional hospitals, Johns Hopkins All Children’s brings care closer to home. Johns Hopkins All Children’s Hospital consistently keeps the patient and family at the center of care while continuing to expand its mission in treatment, research, education and advocacy. For more information, visit HopkinsAllChildrens.org .
Diabetes breakthrough: Scientists uncover novel route to stimulate beta cell growth
- Download PDF Copy
Researchers at Weill Cornell Medicine have uncovered a novel route to stimulate the growth of healthy insulin-producing pancreatic beta cells in a preclinical model of diabetes. The findings hold promise for future therapeutics that will improve the lives of individuals with type 2 diabetes—a condition that affects more than half a billion people worldwide.
This study, published in the Journal of Clinical Investigation on Sept. 15, demonstrated that activating a pathway to promote cell division not only expanded the population of insulin-producing cells, but, surprisingly, it also enhanced the cells' function.
That's reassuring because there is a long-standing belief in the field that proliferation can lead to 'de-differentiation' and a loss of cell function. Our result flies in the face of that dogma and suggests if we can find a way to trigger replication of the beta cells in the body, we won't impair their ability to produce and secrete insulin." Dr. Laura Alonso, study senior author, chief of the division of endocrinology, diabetes and metabolism, director of the Weill Center for Metabolic Health, and the E. Hugh Luckey Distinguished Professor in Medicine at Weill Cornell Medicine
First author, Rachel Stamateris, also contributed to this work as an MD, PhD student at the University of Massachusetts Medical School and visiting graduate assistant in medicine at Weill Cornell Medicine.
When beta cells fail
In type 2 diabetes, which is typically associated with obesity, the body's tissues become resistant to insulin, which means they can't take in and use blood sugar. At the same time, insulin-producing beta cells in the pancreas fail—diminishing in number and losing their ability to function.
Dr. Alonso and her colleagues reproduced these conditions in a mouse model of diabetes that lacks IRS2, a protein that allows insulin to transmit its signal for cells to absorb blood sugar. These mice displayed insulin resistance, a seminal feature of human type 2 diabetes. "On top of that," said Dr. Alonso, "the IRS2 protein also turns out to be critical for beta cell function and beta cell number." So, their pool of beta cells was depleted.
Related Stories
- Older adults with diabetes faced elevated risk of depression during the COVID-19 pandemic
- Xanthones show promise in diabetes with antioxidant and anti-inflammatory properties
- Study reveals reduced type 1 diabetes risk in post-10-year-old girls
The first order of business to rescue these mice: boost beta cell numbers. But how? She and her team took a closer look at the molecular machinery that controls cell proliferation. The researchers observed that in the IRS-deficient diabetic mice, beta cells failed to elevate production of cyclin D2. This protein, when partnered with a protein called CDK4, drives cell division. Previous studies had shown that mice lacking CDK4 also develop diabetes.
It seemed logical to test if boosting CDK4 activity would help increase beta cell quantity.
Beta cell proliferation—quantity and quality
When Dr. Alonso's team genetically introduced an active form of CDK4 into the diabetic mice that was more available to attach to cyclin D2, the first thing they noticed was the animals' blood sugars were restored to normal. Their beta cells were more plentiful than in the untreated, IRS2 mutant mice. But even better: "The beta cells looked amazingly healthy in the treated mice compared with the original diabetic mice, whose beta cells look terrible. Increasing the activity of CDK4 resulted in beta cells packed full of insulin," said Dr. Alonso, who is also an endocrinologist at NewYork-Presbyterian/Weill Cornell Medical Center. This supports the concept that beta cell mass can be expanded without compromising function.
While CDK4 is not, itself, a viable therapeutic target because its ability to stimulate proliferation could increase the risk of cancer, Dr. Alonso is confident that probing the molecular pathways that govern beta cell division and function could someday lead to a clinical breakthrough. She pointed to Ozempic, one of the most talked about new treatments for diabetes. "That medication was discovered by a scientist studying toxins in the saliva of the Gila monster," said Dr. Alonso. "So, it's clear that understanding how fundamental biology works can lead to real advances in treating or even preventing diabetes."
Weill Cornell Medicine
Stamateris, R. E., et al . (2023). Noncanonical CDK4 signaling rescues diabetes in a mouse model by promoting β cell differentiation. The Journal of Clinical Investigation . doi.org/10.1172/JCI166490 .
Posted in: Medical Research News | Medical Condition News
Tags: Blood , Blood Sugar , Cancer , Cell , Cell Division , Cell Proliferation , Diabetes , Endocrinologist , Endocrinology , Insulin , Insulin Resistance , Kidney , Medical School , Medicine , Metabolism , Mouse Model , Obesity , Pancreas , Preclinical , Proliferation , Protein , Semaglutide , Therapeutics , Toxins , Type 2 Diabetes
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

A Discussion with Hologic’s Tim Simpson on the Future of Cervical Cancer Screening
Tim Simpson
Hologic’s Tim Simpson Discusses the Future of Cervical Cancer Screening.

From Waste to Taste: The Transformative Power of Fermented Foods
Maria Marco
In this interview conducted at Pittcon 2024 in San Diego, Maria Marco discusses her research on the health benefits, safety, and waste reduction potential of fermented foods, and the microbial processes involved in their production.

Revolutionizing hepatocyte count for researchers
Dan Schieffer
In this interview, NewsMedical speaks to DeNovix's Dan Schieffer about the new app developed for CellDrop, and how it aims to revolutionize hepatocyte count for the drug discovery industry, and more.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- SPONSOR FEATURE Sponsor retains sole responsibility for the content of this article
Diabetes: Following the science in the search for a cure
Produced by
Diabetes is one of the priority cardiovascular, renal and metabolic diseases for which AstraZeneca is developing novel therapies. The global number of people living with diabetes is expected to rise to 700 million by 2045 1 . AstraZeneca’s ambition is not just to reduce the raised blood glucose typical of the disease, it is to tackle the underlying pathophysiological drivers in order to instigate diabetes remission, prevent diabetic complications and deaths and, ultimately, to deliver a cure.
“AstraZeneca recognises that diabetes is more than a disease of the pancreas, and our research is focused on the underlying mechanisms that link diabetes to comorbidities, especially the main causes of death from the disease, including myocardial infarction and stroke, as well as heart and kidney failure,” says David Baker, Head of Metabolism Bioscience, Biopharmaceuticals R&D.
Induce remission
New approaches for the treatment of type 2 diabetes aim to induce remission as soon as possible after diagnosis by achieving durable responses to novel medicines designed to target the causes of type 2 diabetes and its related complications.
Obesity and insulin resistance are two key drivers in the development of type 2 diabetes, and are also connected to the development of diseases of the heart and circulation, liver and kidneys.
In people with obesity, bariatric surgery can cause remission of type 2 diabetes, and therapeutic alternatives are being sought to achieve levels of weight loss comparable to surgery 2 . Insulin resistance (reduced cell response to the glucose-lowering pancreatic hormone, insulin) has been a target for anti-diabetic medicines for many years. However, growing understanding of its central role in the development of blood vessel, kidney and liver diseases, has made it a research priority for addressing a key gap in the therapeutic armoury available to doctors and patients.
Precision medicine
There is no single gene for type 2 diabetes but identification of the genomic drivers of the multiple biological mechanisms that lead to diabetes holds considerable promise. It is expected that new genomic understanding, linked to clinical phenotypes, will help to differentiate sub-groups of patients according to their risk of developing diabetes and progressing to diabetic complications. This segmentation of type 2 diabetes could also indicate opportunities for targeted therapies to intervene early in the disease.
Novel therapeutics
Advancing our understanding of disease biology enables us to uncover potential novel approaches for future diabetes treatments. At AstraZeneca, our toolbox of drug modalities enables us to address almost any drug target in diabetes using a range of therapeutics from classical small drug molecules to novel agents such as oligonucleotide and mRNA therapies. Approaches are being developed that can repair or modify a cell’s blueprint for making key proteins that can be applied to type 2 diabetes and its complications.
Antisense oligonucleotides are short, synthetic, chemically modified pieces of RNA that can be used to ‘silence’ genes and prevent detrimental proteins from being made. Antisense oligonucleotides have been designed for delivery specifically into the insulin-producing beta-cells of the pancreas whose dysfunction can lead to diabetes 2 . This has opened the door to antisense oligonucleotide therapy aimed at restoring beta-cell function in diabetes and potentially ‘knocking out’ other genes in a way that could support long-term remission or even cure ( Fig. 1 ).

Figure 1. Pancreactic beta cells at different stages of regeneration.
Messenger RNA (mRNA) has also been investigated for its potential in treating the small blood vessel damage, which commonly occurs with diabetes and can lead to organ failure. Encouraging results were obtained with injections of modified mRNA encoding for vascular endothelial growth factor A (VEGF-A), a protein involved in blood vessel formation. Local expression of VEGF-A was accompanied by improved blood flow in the skin of men with type 2 diabetes 3 .
Making insulin-resistant cells more sensitive to insulin is another goal of novel therapeutics for diabetes. In preclinical research, insulin sensitivity has been improved with gene therapy, antisense oligonucleotides and novel biologic approaches to decrease ectopic fat, particularly in the liver, which in turn alleviates insulin resistance and rejuvenates beta-cell function, leading to diabetes remission.
From remission to cure
If we want to change the trajectory of rising diabetes cases, we need to use novel approaches that target the drivers of diseases.At AstraZeneca, we are using our genomics expertise and state-of-the-art drug discovery technologies to build on strong science to develop potential medicines aimed at treating – and ultimately curing – diabetes.
Christopher Rhodes 1 , David Baker 2 & Regina Danielson Fritsche 3 .
1. One MedImmune Way, Gaithersburg, Maryland 20878, US.
2. Granta Park, Great Abington, Cambridge, CB21 6GH, UK.
3. Pepparedsleden 1, Mölndal, SE-431 83, Sweden.
International Diabetes Federation. Diabetes Atlas (2019).
Ämmälä, C., et al. Sci. Adv. 4 , eaat3386 (2018).
Article PubMed Google Scholar
Gan, L.-M., et al. Nat Commun 10 , 871 (2019).
Download references
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Summary: Researchers have identified an enzyme that blocks insulin produced in the body -- a discovery that could provide a new target to treat diabetes. The study focuses on nitric oxide, a...
Scientists at Mount Sinai and City of Hope medical centres in the US have developed a drug treatment which was able to increase insulin-producing cells - known as beta cells - by 700 per cent...
Research published by Baker Heart and Diabetes Institute scientists shows they have manipulated existing pancreatic stem cells to prompt them to produce insulin. The study from the Melbourne researchers builds on previous work by Monash University scientists, using two existing cancer drugs.
Targeting the inceptor receptor could lead to breakthrough treatments for diabetes by protecting beta cells and improving blood sugar control, with German research institutions leading this promising discovery.
For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease.
The new research, published in the journal Nature Communications, offers a potential strategy for developing new therapies that could restore dysfunctional pancreatic beta-cells or, perhaps, even prevent Type 2 diabetes from developing.
In two newly published papers in Cell Reports, Renquist, along with researchers from Washington University in St. Louis, the University of Pennsylvania and Northwestern University, outline a...
Many people with diabetes live under the fear of a slip-up — an over- or under-dose that could end in a seizure, coma or death. Insulin is a lifeline, but it’s not a cure. Five researchers at the Alberta Diabetes Institute (ADI) are bringing us closer to a cure than we’ve ever been.
Researchers at Weill Cornell Medicine have uncovered a novel route to stimulate the growth of healthy insulin-producing pancreatic beta cells in a preclinical model of diabetes.
New approaches for the treatment of type 2 diabetes aim to induce remission as soon as possible after diagnosis by achieving durable responses to novel medicines designed to target the causes of...