Interpersonal Psychotherapy (IPT) for PTSD: A Case Study
Information & authors, metrics & citations, view options, introduction, empirical support, trauma history, presenting complaints, hyperarousal symptoms, avoidance/numbing symptoms, intrusive symptoms, other symptoms, previous treatments, treatment overview, sessions 1 to 3: initial phase, ipt case formulation.
I understand from our initial meeting that your interpersonal goals are to be closer to Chloe and to reduce disputes with Diane. I also understand that you’ve always experienced interpersonal difficulties, but that they grew much worse after your daughter’s abduction-triggered PTSD. You have clearly worked hard over the years to overcome problems you’ve had in social arenas, and you’ve tried numerous times to address the painful memories of past traumas that still live with you today . Your PTSD symptoms still overshadow your feelings and actions. You feel overwhelmed by both your emotions and your environment. Your symptoms are also coupled with an important current life issue you say has you’ve never discussed in past treatments: your sexual identity. Through understanding yourself in relationship to others, you cam mend your social conflicts and reduce your symptoms . You’ve discussed how hard it is to trust people, and how that has limited your social network for years. Your wife’s abduction of Chloe took away the person closest in the world to you, and has made it extremely difficult—to this day—for you to trust others, to take the risk to connect with those around you. This mistrust is very common in PTSD. Avoidance, numbing, intrusive thoughts are all symptoms of the illness. Although you say that you always had difficulty in social situations, these symptoms are not necessarily part of your character; they’re indication of an illness that you suffer from—an illness that’s treatable and not your fault. The symptoms can improve . Your mistrust has led you to minimize social contact. You’ve discussed feeling “betrayed ” or “deceived ” after trying to help others many times over . So you’ve been keeping your distance through “ electronic relationships ” that are more comfortable. Yet, you say you “yearn for closer, more real relationships!” You are going through a role transition: Uncomfortable feelings about your relationships and your own sexuality have made life extremely confusing, and it’s hard for you to know what you want from whom. What we can work on in the remaining weeks of treatment is how to navigate this transition: Do you want to stay with Diane, deepen a relationship with Jane, or what? If you can understand your feelings and use them to resolve this uncertainty, not only will your life feel better, but you symptoms are likely to subside. Does that make sense to you?

Session 4 to 10: Middle Phase
Session 11-14: termination phase, assessment of progress, complicating factors during the course of treatment, treatment implications, acknowledgments, information, published in.

- Posttraumatic stress disorder
- interpersonal psychotherapy
- affect dysregulation
- interpersonal difficulties
Affiliations
Export citations.
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download. For more information or tips please see 'Downloading to a citation manager' in the Help menu .
| Format | |
|---|---|
| Citation style | |
| Style | |
To download the citation to this article, select your reference manager software.
There are no citations for this item
View options
Login options.
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Purchase Options
Purchase this article to access the full text.
PPV Articles - APT - American Journal of Psychotherapy
Not a subscriber?
Subscribe Now / Learn More
PsychiatryOnline subscription options offer access to the DSM-5-TR ® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
Share article link
Copying failed.
PREVIOUS ARTICLE
Next article, request username.
Can't sign in? Forgot your username? Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username
Create a new account
Change password, password changed successfully.
Your password has been changed
Reset password
Can't sign in? Forgot your password?
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password.
Your Phone has been verified
As described within the American Psychiatric Association (APA)'s Privacy Policy and Terms of Use , this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences. Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 29 March 2022
Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits
- Kerry. J. Ressler ORCID: orcid.org/0000-0002-5158-1103 1 ,
- Sabina Berretta 1 ,
- Vadim Y. Bolshakov 1 ,
- Isabelle M. Rosso 1 ,
- Edward G. Meloni 1 ,
- Scott L. Rauch 1 &
- William A. Carlezon Jr 1
Nature Reviews Neurology volume 18 , pages 273–288 ( 2022 ) Cite this article
10k Accesses
130 Citations
170 Altmetric
Metrics details
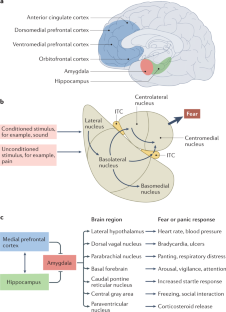
Post-traumatic stress disorder (PTSD) is a maladaptive and debilitating psychiatric disorder, characterized by re-experiencing, avoidance, negative emotions and thoughts, and hyperarousal in the months and years following exposure to severe trauma. PTSD has a prevalence of approximately 6–8% in the general population, although this can increase to 25% among groups who have experienced severe psychological trauma, such as combat veterans, refugees and victims of assault. The risk of developing PTSD in the aftermath of severe trauma is determined by multiple factors, including genetics — at least 30–40% of the risk of PTSD is heritable — and past history, for example, prior adult and childhood trauma. Many of the primary symptoms of PTSD, including hyperarousal and sleep dysregulation, are increasingly understood through translational neuroscience. In addition, a large amount of evidence suggests that PTSD can be viewed, at least in part, as a disorder that involves dysregulation of normal fear processes. The neural circuitry underlying fear and threat-related behaviour and learning in mammals, including the amygdala–hippocampus–medial prefrontal cortex circuit, is among the most well-understood in behavioural neuroscience. Furthermore, the study of threat-responding and its underlying circuitry has led to rapid progress in understanding learning and memory processes. By combining molecular–genetic approaches with a translational, mechanistic knowledge of fear circuitry, transformational advances in the conceptual framework, diagnosis and treatment of PTSD are possible. In this Review, we describe the clinical features and current treatments for PTSD, examine the neurobiology of symptom domains, highlight genomic advances and discuss translational approaches to understanding mechanisms and identifying new treatments and interventions for this devastating syndrome.
Post-traumatic stress disorder (PTSD) is a debilitating neuropsychiatric disorder, characterized by re-experiencing, avoidance, negative emotions and thoughts, and hyperarousal.
PTSD is frequently comorbid with neurological conditions such as traumatic brain injury, post-traumatic epilepsy and chronic headaches.
PTSD has a prevalence of approximately 6–8% in the general population and up to 25% among individuals who have experienced severe trauma.
Many of the neural circuit mechanisms that underlie the PTSD symptoms of fear-related and threat-related behaviour, hyperarousal and sleep dysregulation are becoming increasingly clear.
Key brain regions involved in PTSD include the amygdala–hippocampus–prefrontal cortex circuit, which is among the most well-understood networks in behavioural neuroscience.
Combining molecular–genetic approaches with a mechanistic knowledge of fear circuitry will enable transformational advances in the conceptual framework, diagnosis and treatment of PTSD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders
Impaired learning, memory, and extinction in posttraumatic stress disorder: translational meta-analysis of clinical and preclinical studies
Prefrontal cortex, amygdala, and threat processing: implications for PTSD
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Publishing, 2013).
Breslau, N. et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit area survey of trauma. Arch. Gen. Psychiatry 55 , 626–632 (1998).
Article CAS PubMed Google Scholar
Breslau, N., Peterson, E. L., Poisson, L. M., Schultz, L. R. & Lucia, V. C. Estimating post-traumatic stress disorder in the community: lifetime perspective and the impact of typical traumatic events. Psychol. Med. 34 , 889–898 (2004).
Bromet, E., Sonnega, A. & Kessler, R. C. Risk factors for DSM-III-R posttraumatic stress disorder: findings from the National Comorbidity Survey. Am. J. Epidemiol. 147 , 353–361 (1998).
McLaughlin, K. A. et al. Subthreshold posttraumatic stress disorder in the world health organization world mental health surveys. Biol. Psychiatry 77 , 375–384 (2015).
Article PubMed Google Scholar
Jovanovic, T., Kazama, A., Bachevalier, J. & Davis, M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62 , 695–704 (2011).
Article PubMed PubMed Central CAS Google Scholar
Jovanovic, T. & Ressler, K. J. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry 167 , 648–662 (2010).
Article PubMed PubMed Central Google Scholar
Morgan, C. A., Grillon, C., Southwick, S. M., Davis, M. & Charney, D. S. Fear-potentiated startle in posttraumatic stress disorder. Biol. Psychiatry 38 , 378–385 (1995).
Rauch, S. L. et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol. Psychiatry 47 , 769–776 (2000).
Shin, L. M. et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch. Gen. Psychiatry 62 , 273–281 (2005).
Mellman, T. A., Pigeon, W. R., Nowell, P. D. & Nolan, B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J. Trauma. Stress 20 , 893–901 (2007).
Yehuda, R. & LeDoux, J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56 , 19–32 (2007).
Koenen, K. C., Goodwin, R., Struening, E., Hellman, F. & Guardino, M. Posttraumatic stress disorder and treatment seeking in a national screening sample. J. Trauma. Stress 16 , 5–16 (2003).
Koenen, K. C. et al. A high risk twin study of combat-related PTSD comorbidity. Twin Res. 6 , 218–226 (2003).
True, W. R. et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch. Gen. Psychiatry 50 , 257–264 (1993).
Stein, M. B. et al. Genome-wide association studies of posttraumatic stress disorder in 2 cohorts of US Army soldiers. JAMA Psychiatry 73 , 695–704 (2016).
Stein, M. B., Jang, K. L., Taylor, S., Vernon, P. A. & Livesley, W. J. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am. J. Psychiatry 159 , 1675–1681 (2002).
Duncan, L. E. et al. Largest GWAS of PTSD ( N =20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol. Psychiatry 23 , 666–673 (2018).
Reuveni, I. et al. Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum. Brain Mapp. 37 , 589–599 (2015).
Porter, B., Bonanno, G. A., Frasco, M. A., Dursa, E. K. & Boyko, E. J. Prospective post-traumatic stress disorder symptom trajectories in active duty and separated military personnel. J. Psychiatr. Res. 89 , 55–64 (2017).
Ballenger, J. C. et al. Consensus statement update on posttraumatic stress disorder from the international consensus group on depression and anxiety. J. Clin. Psychiatry 65 (Suppl. 1), 55–62 (2004).
PubMed Google Scholar
Heim, C. & Nemeroff, C. B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry 49 , 1023–1039 (2001).
Kessler, R. C. et al. Trauma and PTSD in the WHO world mental health surveys. Eur. J. Psychotraumatol. 8 , 1353383 (2017).
Huckins, L. M. et al. Analysis of genetically regulated gene expression identifies a prefrontal PTSD gene, SNRNP35, specific to military cohorts. Cell Rep. 31 , 107716 (2020).
Article CAS PubMed PubMed Central Google Scholar
Koenen, K. C. et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med. 47 , 2260–2274 (2017).
Kornfield, S. L., Hantsoo, L. & Epperson, C. N. What does sex have to do with it? The role of sex as a biological variable in the development of posttraumatic stress disorder. Curr. Psychiatry Rep. 20 , 39 (2018).
Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M. & Nelson, C. B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52 , 1048–1060 (1995).
Davis, M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 15 , 353–375 (1992).
Davis, M. in The Amygdala , Second Edition: A Functional Analysis (ed. Aggleton, J. P.) 213–287 (Oxford Univ. Press, 2000).
LeDoux, J. E., Cicchetti, P., Xagoraris, A. & Romanski, L. M. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J. Neurosci. 10 , 1062–1069 (1990).
Maren, S. The amygdala, synaptic plasticity, and fear memory. Ann. NY Acad. Sci. 985 , 106–113 (2003).
Pitkanen, A. in The Amygdala, Second Edition: A Functional Analysis (ed. Aggleton, J. P.) 31–116 (Oxford Univ. Press, 2000).
Milad, M. R. & Quirk, G. J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420 , 70–74 (2002).
McCullough, K. M., Morrison, F. G. & Ressler, K. J. Bridging the gap: towards a cell-type specific understanding of neural circuits underlying fear behaviors. Neurobiol. Learn. Mem. 135 , 27–39 (2016).
Mobbs, D. et al. Viewpoints: approaches to defining and investigating fear. Nat. Neurosci. 22 , 1205–1216 (2019).
Ressler, K. J. Translating across circuits and genetics toward progress in fear- and anxiety-related disorders. Am. J. Psychiatry 177 , 214–222 (2020).
Fenster, R. J., Lebois, L. A. M., Ressler, K. J. & Suh, J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat. Rev. Neurosci. 19 , 535–551 (2018).
McAllister, T. W. Psychopharmacological issues in the treatment of TBI and PTSD. Clin. Neuropsychol. 23 , 1338–1367 (2009).
Strawn, J. R., Keeshin, B. R., DelBello, M. P., Geracioti, T. D. Jr. & Putnam, F. W. Psychopharmacologic treatment of posttraumatic stress disorder in children and adolescents: a review. J. Clin. Psychiatry 71 , 932–941 (2010).
Abdallah, C. G., Southwick, S. M. & Krystal, J. H. Neurobiology of posttraumatic stress disorder (PTSD): a path from novel pathophysiology to innovative therapeutics. Neurosci. Lett. 649 , 130–132 (2017).
Stojek, M. M., McSweeney, L. B. & Rauch, S. A. M. Neuroscience informed prolonged exposure practice: increasing efficiency and efficacy through mechanisms. Front. Behav. Neurosci. 12 , 281 (2018).
Kelmendi, B., Adams, T. G., Soutwick, S., Abdallah, C. G. & Krystal, J. H. Posttraumatic stress disorder: an integrated overview and neurobiological rationale for pharmacology. Clin. Psychol. 24 , 281–297 (2017).
Google Scholar
Krystal, J. H. et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol. Psychiatry 82 , e51–e59 (2017).
Stein, D. J. et al. Dissociation in posttraumatic stress disorder: evidence from the world mental health surveys. Biol. Psyhiatry 73 , 302–312 (2013).
Article Google Scholar
Lebois, L. A. M. et al. Large-scale functional brain network architecture changes associated with trauma-related dissociation. Am. J. Psychiatry 178 , 165–173 (2021).
Nicholson, A. A. et al. Dynamic causal modeling in PTSD and its dissociative subtype: bottom-up versus top-down processing within fear and emotion regulation circuitry. Hum. Brain Mapp. 38 , 5551–5561 (2017).
Stein, M. B. et al. Genome-wide analyses of psychological resilience in U.S. Army soldiers. Am. J. Med. Genet. B Neuropsychiatr. Genet. 180 , 310–319 (2019).
Nievergelt, C. M. et al. Genomic predictors of combat stress vulnerability and resilience in U.S. Marines: a genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology 51 , 459–471 (2015).
Thompson, N. J., Fiorillo, D., Rothbaum, B. O., Ressler, K. J. & Michopoulos, V. Coping strategies as mediators in relation to resilience and posttraumatic stress disorder. J. Affect. Disord. 225 , 153–159 (2018).
van Rooij, S. J. H. et al. Hippocampal activation during contextual fear inhibition related to resilience in the early aftermath of trauma. Behav. Brain Res. 408 , 113282 (2021).
Wingo, A. P., Ressler, K. J. & Bradley, B. Resilience characteristics mitigate tendency for harmful alcohol and illicit drug use in adults with a history of childhood abuse: a cross-sectional study of 2024 inner-city men and women. J. Psychiatr. Res. 51 , 93–99 (2014).
Wrenn, G. L. et al. The effect of resilience on posttraumatic stress disorder in trauma-exposed inner-city primary care patients. J. Natl Med. Assoc. 103 , 560–566 (2011).
Astill Wright, L., Horstmann, L., Holmes, E. A. & Bisson, J. I. Consolidation/reconsolidation therapies for the prevention and treatment of PTSD and re-experiencing: a systematic review and meta-analysis. Transl. Psychiatry 11 , 453 (2021).
Linnstaedt, S. D., Zannas, A. S., McLean, S. A., Koenen, K. C. & Ressler, K. J. Literature review and methodological considerations for understanding circulating risk biomarkers following trauma exposure. Mol. Psychiatry 25 , 1986–1999 (2020).
McLean, S. A. et al. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol. Psychiatry 25 , 283–296 (2020).
Iyadurai, L. et al. Preventing intrusive memories after trauma via a brief intervention involving Tetris computer game play in the emergency department: a proof-of-concept randomized controlled trial. Mol. Psychiatry 23 , 674–682 (2018).
Rothbaum, B. O. et al. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J. Clin. Psychiatry 75 , 1380–1387 (2014).
Seal, K. H. & Stein, M. B. Preventing the pain of PTSD. Sci. Transl Med. 5 , 188fs122 (2013).
Article CAS Google Scholar
Zohar, J. et al. Secondary prevention of chronic PTSD by early and short-term administration of escitalopram: a prospective randomized, placebo-controlled, double-blind trial. J. Clin. Psychiatry 79 , 16m10730 (2018).
Zohar, J. et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur. Neuropsychopharmacol. 21 , 796–809 (2011).
Myers, K. M. & Davis, M. Mechanisms of fear extinction. Mol. Psychiatry 12 , 120–150 (2007).
Ross, D. A. et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry 74 , 407–415 (2017).
LaBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux, J. E. & Phelps, E. A. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20 , 937–945 (1998).
LeDoux, J. The amygdala. Curr. Biol. 17 , R868–R874 (2007).
Rogan, M. T., Staubli, U. V. & LeDoux, J. E. Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390 , 604–607 (1997).
Myers, K. M. & Davis, M. Behavioral and neural analysis of extinction. Neuron 36 , 567–584 (2002).
Chhatwal, J. P., Myers, K. M., Ressler, K. J. & Davis, M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J. Neurosci. 25 , 502–506 (2005).
Herry, C. et al. Switching on and off fear by distinct neuronal circuits. Nature 454 , 600–606 (2008).
Lee, J., An, B. & Choi, S. Longitudinal recordings of single units in the basal amygdala during fear conditioning and extinction. Sci. Rep. 11 , 11177 (2021).
McCullough, K. M. et al. Molecular characterization of Thy1 expressing fear-inhibiting neurons within the basolateral amygdala. Nat. Commun. 7 , 13149 (2016).
Jasnow, A. M. et al. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J. Neurosci. 33 , 10396–10404 (2013).
Hinrichs, R. et al. Increased skin conductance response in the immediate aftermath of trauma predicts PTSD risk. Chronic Stress 3 , 1–11 (2019).
Milad, M. R. et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 66 , 1075–1082 (2009).
Etkin, A. & Wager, T. D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164 , 1476–1488 (2007).
Steuber, E. R. et al. Thalamic volume and fear extinction interact to predict acute posttraumatic stress severity. Psychiatry Res. 141 , 325–332 (2021).
Kredlow, M. A., Fenster, R. J., Laurent, E. S., Ressler, K. J. & Phelps, E. A. Prefrontal cortex, amygdala and threat processing: implications for PTSD. Neuropsychopharmacology 47 , 247–259 (2022).
Maddox, S. A., Hartmann, J., Ross, R. A. & Ressler, K. J. Deconstructing the gestalt: mechanisms of fear, threat, and trauma memory encoding. Neuron 102 , 60–74 (2019).
Tsvetkov, E., Carlezon, W. A., Benes, F. M., Kandel, E. R. & Bolshakov, V. Y. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron 34 , 289–300 (2002).
Josselyn, S. A. et al. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J. Neurosci. 21 , 2404–2412 (2001).
Bremner, J. D. et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am. J. Psychiatry 152 , 973–981 (1995).
Bremner, J. D. et al. The environment contributes more than genetics to smaller hippocampal volume in posttraumatic stress disorder (PTSD). J. Psychiatr. Res. 137 , 579–588 (2021).
Logue, M. W. et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol. Psychiatry 83 , 244–253 (2018).
Heldt, S. A., Stanek, L., Chhatwal, J. P. & Ressler, K. J. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 12 , 656–670 (2007).
Ji, J. & Maren, S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn. Mem. 12 , 270–276 (2005).
Parsons, R. G. & Ressler, K. J. Implications of memory modulation for post-traumatic stress and fear disorders. Nat. Neurosci. 16 , 146–153 (2013).
Brown, E. S., Rush, A. J. & McEwen, B. S. Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology 21 , 474–484 (1999).
Herrmann, L. et al. Long-lasting hippocampal synaptic protein loss in a mouse model of posttraumatic stress disorder. PLoS ONE 7 , e42603 (2012).
Hobin, J. A., Goosens, K. A. & Maren, S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J. Neurosci. 23 , 8410–8416 (2003).
Peters, J., Dieppa-Perea, L. M., Melendez, L. M. & Quirk, G. J. Induction of fear extinction with hippocampal-infralimbic BDNF. Science 328 , 1288–1290 (2010).
Milad, M. R. et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 62 , 446–454 (2007).
Milad, M. R. et al. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc. Natl Acad. Sci. USA 102 , 10706–10711 (2005).
Santhanam, P. et al. Decreases in white matter integrity of ventro-limbic pathway linked to post-traumatic stress disorder in mild traumatic brain injury. J. Neurotrauma 36 , 1093–1098 (2019).
Koch, S. B. J. et al. Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: a diffusion tensor imaging study. J. Psychiatry Neurosci. 42 , 331–342 (2017).
Kennis, M., van Rooij, S. J. H., Reijnen, A. & Geuze, E. The predictive value of dorsal cingulate activity and fractional anisotropy on long-term PTSD symptom severity. Depress. Anxiety 34 , 410–418 (2017).
Sotres-Bayon, F., Sierra-Mercado, D., Pardilla-Delgado, E. & Quirk, G. J. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76 , 804–812 (2012).
Lanius, R. A., Brand, B., Vermetten, E., Frewen, P. A. & Spiegel, D. The dissociative subtype of posttraumatic stress disorder: rationale, clinical and neurobiological evidence, and implications. Depress. Anxiety 29 , 701–708 (2012).
Mellman, T. A. & Hipolito, M. M. Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectr. 11 , 611–615 (2006).
Neylan, T. C. et al. Prior sleep problems and adverse post-traumatic neuropsychiatric sequelae of motor vehicle collision in the AURORA study. Sleep 44 , zsaa200 (2021).
van Liempt, S., van Zuiden, M., Westenberg, H., Super, A. & Vermetten, E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety 30 , 469–474 (2013).
Pruiksma, K. E. et al. Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychol. Trauma. 8 , 697–701 (2016).
Bryant, R. A., O’Donnell, M. L., Creamer, M., McFarlane, A. C. & Silove, D. Posttraumatic intrusive symptoms across psychiatric disorders. J. Psychiatr. Res. 45 , 842–847 (2011).
Phelps, A. J. et al. Polysomnography study of the post-traumatic nightmares of post-traumatic stress disorder. Sleep 41 , zsx188 (2018).
Harb, G. C. et al. A critical review of the evidence base of imagery rehearsal for posttraumatic nightmares: pointing the way for future research. J. Trauma. Stress 26 , 570–579 (2013).
Norrholm, S. D. et al. Fear load: the psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int. J. Psychophysiol. 98 , 270–275 (2015).
Fani, N. et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol. Med. 42 , 533–543 (2012).
Norrholm, S. D. et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatry 69 , 556–563 (2011).
Colvonen, P. J., Straus, L. D., Acheson, D. & Gehrman, P. A review of the relationship between emotional learning and memory, sleep, and PTSD. Curr. Psychiatry Rep. 21 , 2 (2019).
van Liempt, S. et al. Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology 38 , 155–165 (2013).
Article PubMed CAS Google Scholar
Lipinska, G. & Thomas, K. J. F. Rapid eye movement fragmentation, not slow-wave sleep, predicts neutral declarative memory consolidation in posttraumatic stress disorder. J. Sleep. Res. 28 , e12846 (2019).
Onton, J. A., Matthews, S. C., Kang, D. Y. & Coleman, T. P. In-home sleep recordings in military veterans with posttraumatic stress disorder reveal less REM and deep sleep <1 Hz. Front. Hum. Neurosci. 12 , 196 (2018).
Wells, A. M. et al. Effects of chronic social defeat stress on sleep and circadian rhythms are mitigated by kappa-opioid receptor antagonism. J. Neurosci. 37 , 7656–7668 (2017).
Hasler, B. P., Insana, S. P., James, J. A. & Germain, A. Evening-type military veterans report worse lifetime posttraumatic stress symptoms and greater brainstem activity across wakefulness and REM sleep. Biol. Psychol. 94 , 255–262 (2013).
Nestler, E. J. & Carlezon, W. A. Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59 , 1151–1159 (2006).
McCullough, K. M. et al. Nucleus accumbens medium spiny neuron subtypes differentially regulate stress-associated alterations in sleep architecture. Biol. Psychiatry 89 , 1138–1149 (2021).
Sijbrandij, M., Engelhard, I. M., Lommen, M. J., Leer, A. & Baas, J. M. Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD). J. Psychiatr. Res. 47 , 1991–1997 (2013).
Rothbaum, B. O. & Davis, M. Applying learning principles to the treatment of post-trauma reactions. Ann. NY Acad. Sci. 1008 , 112–121 (2003).
Ressler, K. J. et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470 , 492–497 (2011).
Ladd, C. O., Plotsky, P. M., Davis, M. in Encyclopedia of Stress 2nd edn (ed. George Fink) 561–568 (Academic, 2007).
Davis, M. Sensitization of the acoustic startle reflex by footshock. Behav. Neurosci. 103 , 495–503 (1989).
Grillon, C., Ameli, R., Woods, S. W., Merikangas, K. & Davis, M. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology 28 , 588–595 (1991).
Giardino, W. J. & Pomrenze, M. B. Extended amygdala neuropeptide circuitry of emotional arousal: waking up on the wrong side of the bed nuclei of stria terminalis. Front. Behav. Neurosci. 15 , 613025 (2021).
Hinrichs, R. et al. Mobile assessment of heightened skin conductance in posttraumatic stress disorder. Depress Anxiety 34 , 502–507 (2017).
Neylan, T. C., Schadt, E. E. & Yehuda, R. Biomarkers for combat-related PTSD: focus on molecular networks from high-dimensional data. Eur. J. Psychotraumatol. 5 , 23938 (2014).
Yehuda, R., Giller, E. L., Southwick, S. M., Lowy, M. T. & Mason, J. W. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biol. Psychiatry 30 , 1031–1048 (1991).
Yehuda, R. et al. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am. J. Psychiatry 150 , 83–86 (1993).
Han, X. & Boyden, E. S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2 , e299 (2007).
Dunlop, B. W. et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol. Psychiatry 82 , 866–874 (2017).
Jovanovic, T. et al. Psychophysiological treatment outcomes: corticotropin-releasing factor type 1 receptor antagonist increases inhibition of fear-potentiated startle in PTSD patients. Psychophysiology 57 , e13356 (2020).
Galatzer-Levy, I. R. & Bryant, R. A. 636,120 ways to have posttraumatic stress disorder. Perspect. Psychol. Sci. 8 , 651–662 (2013).
Brewin, C. R. The nature and significance of memory disturbance in posttraumatic stress disorder. Annu. Rev. Clin. Psychol. 7 , 203–227 (2011).
Vasterling, J. J. & Arditte Hall, K. A. Neurocognitive and information processing biases in posttraumatic stress disorder. Curr. Psychiatry Rep. 20 , 99 (2018).
Stevens, J. S. & Jovanovic, T. Role of social cognition in post-traumatic stress disorder: a review and meta-analysis. Genes Brain Behav. 18 , e12518 (2019).
Mathias, J. L. & Mansfield, K. M. Prospective and declarative memory problems following moderate and severe traumatic brain injury. Brain Inj. 19 , 271–282 (2005).
Acosta, S. A. et al. Influence of post-traumatic stress disorder on neuroinflammation and cell proliferation in a rat model of traumatic brain injury. PLoS ONE 8 , e81585 (2013).
Elzinga, B. M. & Bremner, J. D. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect. Disord. 70 , 1–17 (2002).
Germine, L. T. et al. Neurocognition after motor vehicle collision and adverse post-traumatic neuropsychiatric sequelae within 8 weeks: initial findings from the AURORA study. J. Affect. Disord. 298 , 57–67 (2022).
Ben-Zion, Z. et al. Neuroanatomical risk factors for posttraumatic stress disorder in recent trauma survivors. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5 , 311–319 (2020).
Dark, H. E., Harnett, N. G., Knight, A. J. & Knight, D. C. Hippocampal volume varies with acute posttraumatic stress symptoms following medical trauma. Behav. Neurosci. 135 , 71–78 (2021).
van Rooij, S. J. H. et al. The role of the Hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biol. Psychiatry 84 , 106–115 (2018).
Girgenti, M. J. et al. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat. Neurosci. 24 , 24–33 (2021).
Wolf, E. J. et al. Klotho, PTSD, and advanced epigenetic age in cortical tissue. Neuropsychopharmacology 46 , 721–730 (2021).
Olive, I., Makris, N., Densmore, M., McKinnon, M. C. & Lanius, R. A. Altered basal forebrain BOLD signal variability at rest in posttraumatic stress disorder: a potential candidate vulnerability mechanism for neurodegeneration in PTSD. Hum. Brain Mapp. 42 , 3561–3575 (2021).
Mohlenhoff, B. S., O’Donovan, A., Weiner, M. W. & Neylan, T. C. Dementia risk in posttraumatic stress disorder: the relevance of sleep-related abnormalities in brain structure, amyloid, and inflammation. Curr. Psychiatry Rep. 19 , 89 (2017).
Miller, M. W. & Sadeh, N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol. Psychiatry 19 , 1156–1162 (2014).
Mohamed, A. Z., Cumming, P., Gotz, J. & Nasrallah, F., Department of Defense Alzheimer’s Disease Neuroimaging Initiative. Tauopathy in veterans with long-term posttraumatic stress disorder and traumatic brain injury. Eur. J. Nucl. Med. Mol. Imaging 46 , 1139–1151 (2019).
Kaplan, G. B., Vasterling, J. J. & Vedak, P. C. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav. Pharmacol. 21 , 427–437 (2010).
Mojtabavi, H., Saghazadeh, A., van den Heuvel, L., Bucker, J. & Rezaei, N. Peripheral blood levels of brain-derived neurotrophic factor in patients with post-traumatic stress disorder (PTSD): a systematic review and meta-analysis. PLoS One 15 , e0241928 (2020).
Licznerski, P. et al. Decreased SGK1 expression and function contributes to behavioral deficits induced by traumatic stress. PLoS Biol. 13 , e1002282 (2015).
de Kloet, C. S. et al. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J. Psychiatr. Res. 40 , 550–567 (2006).
Hawn, S. E. et al. GxE effects of FKBP5 and traumatic life events on PTSD: a meta-analysis. J. Affect. Disord. 243 , 455–462 (2019).
Zannas, A. S. & Binder, E. B. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. 13 , 25–37 (2014).
Friend, S. F., Nachnani, R., Powell, S. B. & Risbrough, V. B. C-Reactive protein: marker of risk for post-traumatic stress disorder and its potential for a mechanistic role in trauma response and recovery. Eur. J. Neurosci. https://doi.org/10.1111/ejn.15031 (2020).
Michopoulos, V. et al. Association of prospective risk for chronic PTSD symptoms with low TNFα and IFNγ concentrations in the immediate aftermath of trauma exposure. Am. J. Psychiatry 177 , 58–65 (2020).
Michopoulos, V., Powers, A., Gillespie, C. F., Ressler, K. J. & Jovanovic, T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42 , 254–270 (2017).
Michopoulos, V. et al. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatry 172 , 353–362 (2015).
Smith, A. K. et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 156B , 700–708 (2011).
Siegel, C. E. et al. Utilization of machine learning for identifying symptom severity military-related PTSD subtypes and their biological correlates. Transl. Psychiatry 11 , 227 (2021).
Wuchty, S. et al. Integration of peripheral transcriptomics, genomics, and interactomics following trauma identifies causal genes for symptoms of post-traumatic stress and major depression. Mol. Psychiatry 26 , 3077–3092 (2021).
Kuan, P. F. et al. PTSD is associated with accelerated transcriptional aging in World Trade Center responders. Transl. Psychiatry 11 , 311 (2021).
Logue, M. W. et al. An epigenome-wide association study of posttraumatic stress disorder in US veterans implicates several new DNA methylation loci. Clin. Epigenetics 12 , 46 (2020).
Sheerin, C. M. et al. Epigenome-wide study of posttraumatic stress disorder symptom severity in a treatment-seeking adolescent sample. J. Trauma. Stress 34 , 607–615 (2021).
Yang, R. et al. A DNA methylation clock associated with age-related illnesses and mortality is accelerated in men with combat PTSD. Mol. Psychiatry 26 , 4999–5009 (2020).
Smith, A. K. et al. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am. J. Med. Genet. B Neuropsychiatr. Genet. 168B , 36–44 (2015).
Nievergelt, C. M. et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat. Commun. 10 , 4558 (2019).
Gelernter, J. et al. Genome-wide association study of maximum habitual alcohol intake in >140,000 U.S. European and African American veterans yields novel risk loci. Biol. Psychiatry 86 , 365–376 (2019).
Stein, M. B. et al. Genome-wide association analyses of post-traumatic stress disorder and its symptom subdomains in the Million Veteran Program. Nat. Genet. 53 , 174–184 (2021).
Gelernter, J. et al. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat. Neurosci. 22 , 1394–1401 (2019).
Logue, M. W. et al. The Psychiatric Genomics Consortium Posttraumatic Stress Disorder Workgroup: posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology 40 , 2287–2297 (2015).
Pape, J. C. et al. DNA methylation levels are associated with CRF 1 receptor antagonist treatment outcome in women with post-traumatic stress disorder. Clin. Epigenetics 10 , 136 (2018).
Mizuno, Y. More than 20 years of the discovery of Park2. Neurosci. Res. 159 , 3–8 (2020).
Shaltouki, A. et al. Mitochondrial alterations by PARKIN in dopaminergic neurons using PARK2 patient-specific and PARK2 knockout isogenic iPSC lines. Stem Cell Rep. 4 , 847–859 (2015).
Binder, E. B. et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299 , 1291–1305 (2008).
Heim, C., Owens, M. J., Plotsky, P. M. & Nemeroff, C. B. Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol. Bull. 33 , 185–192 (1997).
CAS PubMed Google Scholar
Bremner, J. D. et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatry 154 , 624–629 (1997).
Dias, B. G. & Ressler, K. J. PACAP and the PAC1 receptor in post-traumatic stress disorder. Neuropsychopharmacology 38 , 245–246 (2013).
Ross, R. A. et al. Circulating PACAP peptide and PAC1R genotype as possible transdiagnostic biomarkers for anxiety disorders in women: a preliminary study. Neuropsychopharmacology 45 , 1125–1133 (2020).
Bangasser, D. A. et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry 15 , 896–904 (2010).
Jovanovic, T. et al. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol. Psychiatry 18 , 742–743 (2013).
Miles, O. W. & Maren, S. Role of the bed nucleus of the stria terminalis in PTSD: insights from preclinical models. Front. Behav. Neurosci. 13 , 68 (2019).
Stroth, N., Holighaus, Y., Ait-Ali, D. & Eiden, L. E. PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Ann. NY Acad. Sci. 1220 , 49–59 (2011).
Ramikie, T. S. & Ressler, K. J. Mechanisms of sex differences in fear and posttraumatic stress disorder. Biol. Psychiatry 83 , 876–885 (2018).
Mercer, K. B. et al. Functional evaluation of a PTSD-associated genetic variant: estradiol regulation and ADCYAP1R1. Transl. Psychiatry 6 , e978 (2016).
Klengel, T. et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 16 , 33–41 (2013).
Fani, N. et al. FKBP5 and attention bias for threat: associations with hippocampal function and shape. JAMA Psychiatry 70 , 392–400 (2013).
Galatzer-Levy, I. R. et al. A cross species study of heterogeneity in fear extinction learning in relation to FKBP5 variation and expression: implications for the acute treatment of posttraumatic stress disorder. Neuropharmacology 116 , 188–195 (2017).
Yun, J. Y., Jin, M. J., Kim, S. & Lee, S. H. Stress-related cognitive style is related to volumetric change of the hippocampus and FK506 binding protein 5 polymorphism in post-traumatic stress disorder. Psychol. Med. https://doi.org/10.1017/S0033291720002949 (2020).
Hartmann, J. et al. Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. 35 , 109185 (2021).
Herrmann, L. et al. Analysis of the cerebellar molecular stress response led to first evidence of a role for FKBP51 in brain FKBP52 expression in mice and humans. Neurobiol. Stress 15 , 100401 (2021).
Young, K. A., Thompson, P. M., Cruz, D. A., Williamson, D. E. & Selemon, L. D. BA11 FKBP5 expression levels correlate with dendritic spine density in postmortem PTSD and controls. Neurobiol. Stress 2 , 67–72 (2015).
Abdallah, C. G. et al. The neurobiology and pharmacotherapy of posttraumatic stress disorder. Annu. Rev. Pharmacol. Toxicol. 59 , 171–189 (2019).
Watts, B. V. et al. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J. Clin. Psychiatry 74 , e541–e550 (2013).
Krystal, J. H. et al. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: a randomized trial. JAMA 306 , 493–502 (2011).
Han, C. et al. The potential role of atypical antipsychotics for the treatment of posttraumatic stress disorder. J. Psychiatr. Res. 56 , 72–81 (2014).
Cipriani, A. et al. Comparative efficacy and acceptability of pharmacological treatments for post-traumatic stress disorder in adults: a network meta-analysis. Psychol. Med. 48 , 1975–1984 (2018).
Adamou, M., Puchalska, S., Plummer, W. & Hale, A. S. Valproate in the treatment of PTSD: systematic review and meta analysis. Curr. Med. Res. Opin. 23 , 1285–1291 (2007).
Andrus, M. R. & Gilbert, E. Treatment of civilian and combat-related posttraumatic stress disorder with topiramate. Ann. Pharmacother. 44 , 1810–1816 (2010).
Hertzberg, M. A. et al. A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Boil. Psychiatry 45 , 1226–1229 (1999).
Wang, H. R., Woo, Y. S. & Bahk, W. M. Anticonvulsants to treat post-traumatic stress disorder. Hum. Psychopharmacol. 29 , 427–433 (2014).
Davis, L. L. et al. Divalproex in the treatment of posttraumatic stress disorder: a randomized, double-blind, placebo-controlled trial in a veteran population. J. Clin. Psychopharmacol. 28 , 84–88 (2008).
Lindley, S. E., Carlson, E. B. & Hill, K. A randomized, double-blind, placebo-controlled trial of augmentation topiramate for chronic combat-related posttraumatic stress disorder. J. Clin. Psychopharmacol. 27 , 677–681 (2007).
Taylor, F. B. et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol. Psychiatry 63 , 629–632 (2008).
Raskind, M. A. et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol. Psychiatry 61 , 928–934 (2007).
Khachatryan, D., Groll, D., Booij, L., Sepehry, A. A. & Schütz, C. G. Prazosin for treating sleep disturbances in adults with posttraumatic stress disorder: a systematic review and meta-analysis of randomized controlled trials. Gen. Hosp. Psychiatry 39 , 46–52 (2016).
Brudey, C. et al. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309 , R315–R321 (2015).
Raskind, M. A. et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J. Clin. Psychiatry 61 , 129–133 (2000).
Porter-Stransky, K. A. et al. Noradrenergic transmission at alpha1-adrenergic receptors in the ventral periaqueductal gray modulates arousal. Biol. Psychiatry 85 , 237–247 (2019).
Mallick, B. N., Singh, S. & Pal, D. Role of alpha and beta adrenoceptors in locus coeruleus stimulation-induced reduction in rapid eye movement sleep in freely moving rats. Behav. Brain Res. 158 , 9–21 (2005).
Germain, A. et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J. Psychosom. Res. 72 , 89–96 (2012).
Raskind, M. A. et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am. J. Psychiatry 170 , 1003–1010 (2013).
Raskind, M. A. et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N. Engl. J. Med. 378 , 507–517 (2018).
Dutkiewics, S. Efficacy and tolerability of drugs for treatment of benign prostatic hyperplasia. Int. Urol. Nephrol. 32 , 423–432 (2001).
Lewis, C., Roberts, N. P., Andrew, M., Starling, E. & Bisson, J. I. Psychological therapies for post-traumatic stress disorder in adults: systematic review and meta-analysis. Eur. J. Psychotraumatol. 11 , 1729633 (2020).
Powers, M. B., Halpern, J. M., Ferenschak, M. P., Gillihan, S. J. & Foa, E. B. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin. Psychol. Rev. 30 , 635–641 (2010).
Pavlov, I. P. & Anrep, G. V. Conditioned Reflexes; An Investigation of the Physiological Activity of the Cerebral Cortex (Oxford Univ. Press, 1927).
Singewald, N., Schmuckermair, C., Whittle, N., Holmes, A. & Ressler, K. J. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol. Ther. 149 , 150–190 (2015).
Keynan, J. N. et al. Electrical fingerprint of the amygdala guides neurofeedback training for stress resilience. Nat. Hum. Behav. 3 , 63–73 (2019).
Chen, B. K. et al. Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine. Neuropsychopharmacology 45 , 1545–1556 (2020).
Van’t Veer, A. & Carlezon, W. A. Jr. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology 229 , 435–452 (2013).
Smith, A. K. et al. Epigenome-wide meta-analysis of PTSD across 10 military and civilian cohorts identifies methylation changes in AHRR. Nat. Commun. 11 , 5965 (2020).
Mellon, S. H. et al. Metabolomic analysis of male combat veterans with post traumatic stress disorder. PLoS ONE 14 , e0213839 (2019).
Bremner, J. D. Neuroimaging in posttraumatic stress disorder and other stress-related disorders. Neuroimaging Clin. N. Am. 17 , 523–538 (2007).
Britton, J. C., Phan, K. L., Taylor, S. F., Fig, L. M. & Liberzon, I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol. Psychiatry 57 , 832–840 (2005).
Insel, T. R. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am. J. Psychiatry 171 , 395–397 (2014).
Hyman, S. Mental health: depression needs large human-genetics studies. Nature 515 , 189–191 (2014).
Bale, T. L. et al. The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology 44 , 1349–1353 (2019).
Baker, J. T., Germine, L. T., Ressler, K. J., Rauch, S. L. & Carlezon, W. A. Jr. Digital devices and continuous telemetry: opportunities for aligning psychiatry and neuroscience. Neuropsychopharmacology 43 , 2499–2503 (2018).
Cakmak, A. S. et al. Classification and prediction of post-trauma outcomes related to PTSD using circadian rhythm changes measured via wrist-worn research watch in a large longitudinal cohort. IEEE J. Biomed. Health Inf. 25 , 2866–2876 (2021).
Tsanas, A., Woodward, E. & Ehlers, A. Objective characterization of activity, sleep, and circadian rhythm patterns using a wrist-worn actigraphy sensor: insights into posttraumatic stress disorder. JMIR Mhealth Uhealth 8 , e14306 (2020).
Thompson, R. S. et al. Repeated fear-induced diurnal rhythm disruptions predict PTSD-like sensitized physiological acute stress responses in F344 rats. Acta Physiol. 211 , 447–465 (2014).
Phillips, A. G., Geyer, M. A. & Robbins, T. W. Effective use of animal models for therapeutic development in psychiatric and substance use disorders. Biol. Psychiatry 83 , 915–923 (2018).
Van’t Veer, A., Yano, J. M., Carroll, F. I., Cohen, B. M. & Carlezon, W. A. Jr. Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the kappa-opioid receptor antagonist JDTic. Neuropsychopharmacology 37 , 2809–2816 (2012).
Vogel, S. C. et al. Childhood adversity and dimensional variations in adult sustained attention. Front. Psychol. 11 , 691 (2020).
White, S. F. et al. Increased cognitive control and reduced emotional interference is associated with reduced PTSD symptom severity in a trauma-exposed sample: a preliminary longitudinal study. Psychiatry Res. Neuroimaging 278 , 7–12 (2018).
Beard, C. et al. Abnormal error processing in depressive states: a translational examination in humans and rats. Transl. Psychiatry 5 , e564 (2015).
Robble, M. A. et al. Concordant neurophysiological signatures of cognitive control in humans and rats. Neuropsychopharmacology 46 , 1252–1262 (2021).
Der-Avakian, A. et al. Social defeat disrupts reward learning and potentiates striatal nociceptin/orphanin FQ mRNA in rats. Psychopharmacology 234 , 1603–1614 (2017).
Lokshina, Y., Nickelsen, T. & Liberzon, I. Reward processing and circuit dysregulation in posttraumatic stress disorder. Front. Psychiatry 12 , 559401 (2021).
Lori, A. et al. Transcriptome-wide association study of post-trauma symptom trajectories identified GRIN3B as a potential biomarker for PTSD development. Neuropsychopharmacology 46 , 1811–1820 (2021).
Pacella, M. L., Hruska, B., Steudte-Schmiedgen, S., George, R. L. & Delahanty, D. L. The utility of hair cortisol concentrations in the prediction of PTSD symptoms following traumatic physical injury. Soc. Sci. Med. 175 , 228–234 (2017).
McCullough, K. M. et al. Genome-wide translational profiling of amygdala Crh-expressing neurons reveals role for CREB in fear extinction learning. Nat. Commun. 11 , 5180 (2020).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8 , 1263–1268 (2005).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446 , 633–639 (2007).
Chow, B. Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463 , 98–102 (2010).
Han, X. et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front. Syst. Neurosci. 5 , 18 (2011).
Luchkina, N. V. & Bolshakov, V. Y. Diminishing fear: optogenetic approach toward understanding neural circuits of fear control. Pharmacol. Biochem. Behav. 174 , 64–79 (2018).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141 , 154–165 (2010).
Coward, P. et al. Controlling signaling with a specifically designed Gi-coupled receptor. Proc. Natl Acad. Sci. USA 95 , 352–357 (1998).
Dong, S., Rogan, S. C. & Roth, B. L. Directed molecular evolution of DREADDs: a generic approach to creating next-generation RASSLs. Nat. Protoc. 5 , 561–573 (2010).
Roth, B. L. DREADDs for neuroscientists. Neuron 89 , 683–694 (2016).
Lin, M. Z. & Schnitzer, M. J. Genetically encoded indicators of neuronal activity. Nat. Neurosci. 19 , 1142–1153 (2016).
Download references
Acknowledgements
This work was supported by NIH awards P50-MH115874 (to W.C./K.J.R.), R01-MH108665 (to K.J.R.), R01-MH063266 (to W.C.), R01-MH123993 (to V.Y.B.), and the Frazier Institute at McLean Hospital (to K.J.R.). I.R. was partially supported by (R01-MH120400).
Author information
Authors and affiliations.
SPARED Center, Department of Psychiatry, McLean Hospital, Harvard Medical School, Boston, MA, USA
Kerry. J. Ressler, Sabina Berretta, Vadim Y. Bolshakov, Isabelle M. Rosso, Edward G. Meloni, Scott L. Rauch & William A. Carlezon Jr
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Kerry. J. Ressler .
Ethics declarations
Competing interests.
K.J.R. has received consulting income from Alkermes, Bionomics, Bioxcel and Jazz Pharmaceuticals, and is on scientific advisory boards for the Army STARRS Project, Janssen, the National Center for PTSD, Sage Therapeutics and Verily. He has also received sponsored research support from Brainsway and Takeda. He also serves on the Boards of ACNP and Biological Psychiatry. W.C. has received consulting income from Psy Therapeutics and has a sponsored research agreement with Cerevel Therapeutics. He is the editor-in-chief for Neuropsychopharmacology and serves on the board of ACNP. None of this work is directly related to the work presented here. S.L.R. receives compensation as a Board member of Community Psychiatry and for his role as Secretary of SOBP. He also serves on the Boards of ADAA and NNDC. He has received royalties from Oxford University Press and APPI.
Peer review
Peer review information.
Nature Reviews Neurology thanks Matthew Girgenti, Soraya Seedat and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A core feature of post-traumatic stress disorder (PTSD) that includes irritability, panic and disruptions in sleep and cognitive function.
A reflex that occurs rapidly and unconsciously in response to an external stimulus such as a noise burst.
A core feature of post-traumatic stress disorder (PTSD) characterized by a heightened state of active threat assessment.
A method of inducing alterations in gene expression involving the ability of the enzyme Cre-recombinase to induce site-specific recombination of genetic material.
A theoretical representation of a neural unit of memory storage.
A muscle located in the eyelid, activity of which is often an end point in human fear conditioning research.
Secondary phenotypes that reliably co-occur as a sub-feature of a broader primary phenotype.
Two or more biological processes that are modulated (activated, suppressed) in parallel by a common upstream factor.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Ressler, K.J., Berretta, S., Bolshakov, V.Y. et al. Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol 18 , 273–288 (2022). https://doi.org/10.1038/s41582-022-00635-8
Download citation
Accepted : 18 February 2022
Published : 29 March 2022
Issue Date : May 2022
DOI : https://doi.org/10.1038/s41582-022-00635-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Spatiotemporal dynamics of hippocampal-cortical networks underlying the unique phenomenological properties of trauma-related intrusive memories.
- Kevin J. Clancy
- Quentin Devignes
- Isabelle M. Rosso
Molecular Psychiatry (2024)
Trauma-related intrusive memories and anterior hippocampus structural covariance: an ecological momentary assessment study in posttraumatic stress disorder
Translational Psychiatry (2024)
Advancing preclinical chronic stress models to promote therapeutic discovery for human stress disorders
- Trevonn M. Gyles
- Eric J. Nestler
- Eric M. Parise
Neuropsychopharmacology (2024)
Differential effects of the stress peptides PACAP and CRF on sleep architecture in mice
- Allison R. Foilb
- Elisa M. Taylor-Yeremeeva
- William A. Carlezon
NPP—Digital Psychiatry and Neuroscience (2024)
Memory persistence: from fundamental mechanisms to translational opportunities
- Santiago Abel Merlo
- Mariano Andrés Belluscio
- Emiliano Merlo
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
ORIGINAL RESEARCH article
A brief treatment for veterans with ptsd: an open-label case-series study.

- 1 Department of Clinical Psychology, University of Amsterdam, Amsterdam, Netherlands
- 2 Work Health Technology, The Netherlands Organization for Applied Scientific Research TNO, Leiden, Netherlands
Introduction: Despite the positive outcomes observed in numerous individuals undergoing trauma-focused psychotherapy for PTSD, veterans with this condition experience notably diminished advantages from such therapeutic interventions in comparison to non-military populations.
Methods: In a preliminary study we investigated the efficacy of an innovative treatment approach in a small sample of veterans ( n = 7). Recognizing that accessing and targeting trauma memory in veterans with PTSD may be more challenging compared to other patient populations, we employed unique and personalized retrieval cues that engaged multiple senses and were connected to the context of their trauma. This was followed by a session focused on memory reconsolidation, which incorporated both psychological techniques (i.e., imagery rescripting) and a pharmacological component (i.e., 40 mg of propranolol).
Results: The findings from this small-scale case series cautiously indicate that this brief intervention, typically consisting of only one or two treatment sessions, shows promise in producing significant effects on symptoms of PTSD, distress and quality of life.This is particularly noteworthy given the complex symptomatology experienced by the veterans in this study.
Conclusion: To summarize, there are grounds for optimism regarding this brief treatment of combat-related PTSD. It appears that the potential for positive outcomes is far greater than commonly believed, as demonstrated by the encouraging results of this pilot study.
1. Introduction
The concept that soldiers are tormented by the haunting memories of their wartime ordeals is a recurring theme that echoes through the ages, from the epic verses of Homer’s Iliad, the poetic prose of Shakespeare to the poignant pages of Tolstoy’s ‘war and peace’. Soldiers who were exposed to the trauma of war often experienced a range of symptoms, including anxiety, depression, nightmares, flashbacks, and physical symptoms such as shaking and trembling. Labeling these symptoms as the shell shock syndrome in military personnel during World War I was a significant milestone in the recognition of trauma and its effects on mental health. Initially, these symptoms were often dismissed as signs of weakness or cowardice, and soldiers were sometimes even accused of faking their symptoms to avoid combat. The recognition of shell shock and the subsequent establishment of PTSD as a diagnosis in 1980 (DSM-II) has spurred the creation of effective treatments that have helped many individuals attain recovery and lead satisfying lives. Still, PTSD in past and present members of the military tends to be a chronic condition with prevalence rates varying between 3 and 17% ( 1 – 3 ). Around 80% of those with a diagnosis of PTSD also meets criteria for another mental health condition such as depression, substance use disorder, or another anxiety disorder ( 4 ). While trauma-focused psychotherapy 1 may yield positive results for many individuals suffering from PTSD, veterans with PTSD benefit significantly less than non-military populations ( 5 – 8 ). After receiving trauma-focused psychotherapy for combat-related PTSD, approximately two-thirds of veterans still experience the lingering consequences of this disorder ( 7 , 9 ). Another challenge arises as a considerable number of veterans prematurely end their treatment with dropout rates ranging from 25 to 48% ( 9 , 10 ). Notwithstanding the advances in the field, it is increasingly evident that PTSD continues to be a complex and daunting condition for military personnel and veterans, posing formidable obstacles for traditional trauma-focused psychotherapies. Here, we examine the effectiveness of a novel treatment approach with the goal of mitigating the problem of treatment resistance and the high rates of dropout commonly observed in veterans.
Prior to delving into the details of our innovative treatment approach, we propose several plausible explanations that could clarify the relatively lower effectiveness of trauma-focused interventions in treating veterans with PTSD. Considering that PTSD is viewed as a disturbance of emotional memory (e.g., 11 , 12 ), most psychotherapeutic approaches concentrate on exploring and addressing the individual’s recollection of the traumatic event or its meaning. Regardless of the specific therapeutic approach employed, they all involve revisiting the most distressing and agonizing memories associated with the traumatic experience. These memories, commonly referred to as “hotspot memories” and identified through the intrusive symptoms they elicit, serve as the basis for therapy ( 13 – 17 ). Successful reliving of these memories requires a focus on the sensory details and emotional responses that are integral to the memory ( 12 , 18 , 19 ). While perceptual memory reactivation may not be essential for reducing symptoms, it may facilitate a shift in meaning that ultimately predicts improved treatment outcomes ( 20 ). The general procedure to reactivate trauma memory is to instruct patients to imagine or describe the most disturbing traumatic situations, or the situation that is related to their hotspot memories. Yet, deliberately reliving the battlefield within a therapeutic context does not always guarantee easy access to the emotional intensity of the experience. We must not overlook the fact that military personnel undergo extensive training to equip them with the necessary skills to withstand the arduous physical and mental challenges inherent in their duties. Since emotions such as fear, anger, and sadness can have a significant impact on decision-making and performance in high-stress situations, military training often includes instruction on how to regulate and control their emotions while on duty. Hence, the efficacy of established trauma-focused therapies in evoking a deeply moving emotional response when recalling trauma memories in veterans might pose greater challenges compared to non-military individuals with PTSD. Pinpointing the per-symptom effectiveness of treatments in uncontrolled clinical settings in Israel indeed showed that only a small number of veterans (15.8%, n = 709) experienced minimal relief from symptoms of intrusive traumatic reexperiencing, while two other mnemonic symptoms, namely flashbacks and inability to recall an important aspect of the trauma, exhibited no response to treatment at all in this group of veterans ( 21 ). It is important to mention that not all veterans received trauma-focused therapy in this study. Nonetheless, the failure to effectively address these mnemonic symptoms in veterans may perpetuate other features of the disorder as well ( 21 ).
Another potential limitation is that current therapies were originally designed to address the excessive fear responses to trauma reminders, rather than the multifaceted emotions that may be present in military-related PTSD. Feelings of sadness, anger, shame, guilt, a sense of powerlessness, and betrayal are all common experiences that people may experience when being exposed to trauma ( 22 ). These feelings are particularly intense for members of the military who are often confronted with demanding ethical or moral decisions during their service ( 23 ). While decision-making is often likely to be consistent with their military codes of conduct, substantial levels of psychological distress can still be experienced when they perpetrate, witness or fail to prevent actions that run counter to their core moral or ethical values ( 24 ). This elevated state of distress is identified as ‘moral injury’, a condition closely linked to feelings of guilt, anger and shame ( 25 , 26 ). As a result, individuals with combat-related trauma may not fully benefit from existing treatments, as their emotional needs remain unaddressed. When it comes to addressing the needs of military personnel dealing with PTSD, it’s also essential to consider the emotional toll that therapy can take on veterans. Most trauma-focused therapies require a prolonged course of treatment, which can be emotionally very taxing for military personnel. It is promising though that a recent study has shown a correlation between massed exposure therapy, which consists of delivering a full treatment program over a shorter duration, and a decrease in the number of veterans who discontinue the treatment ( 9 ). Hence, to truly develop more comprehensive and effective treatments for military-related PTSD, further research is necessary to better understand the emotional needs of veterans.
As an initial attempt to overcome the limitations of existing treatments for PTSD, we have developed a novel and concise intervention tailored to combat-related trauma. While most therapies rely on visual and verbal retrieval cues to access emotional memory, we believe that the use of multi-sensory and context-related stimuli as retrieval aids has been surprisingly underutilized. For instance, odors similar to the trauma context could be particularly powerful cues to spontaneously evoke autobiographical memories with a strong emotional resonance ( 27 – 31 ). One advantage of utilizing odors as retrieval cues is that the brain regions involved in olfaction have direct connections to the amygdala and entorhinal cortex ( 32 , 33 ), both of which are involved in emotion processing and memory. To enhance access to emotional memory, we developed idiosyncratic virtual reality worlds that incorporate multi-sensory retrieval cues, including 3D visual, auditory, olfactory, and bodily information, tailored as much as possible to everyone’s personal experiences. After this brief memory reactivation procedure (i.e., 2 min), the focus was shifted toward the idiosyncratic memory representation of the veteran. On the basis of information provided in the intake we had formulated hypothesized stuck points that we tried to directly target in treatment. This part consisted of imaginal exposure combined with rescripting with the rationale that new perspectives on what happened during trauma are most effectively achieved by experiencing new views and emotions which were not possible at the time of the trauma ( 17 , 34 , 35 ). In a previous randomized-controlled trial in nonmilitary-related PTSD, we demonstrated that the addition of imagery rescripting to imaginary exposure led to a significant reduction of treatment dropouts, and better effects on guilt, anger and shame as compared to exposure alone ( 34 ).
Finally, a pivotal modification in the current intervention was to utilize the process of memory reconsolidation as an alternative means of inducing change. Most trauma-focused therapies are rooted on extinction learning with the notable limitation that it can only eliminate the fearful responding while leaving the original trauma memory intact ( 36 , 37 ). As a consequence, the intact trauma memory may resurface thereby explaining the relatively high relapse rates even after initial treatment success ( 38 , 39 ). In contrast, the hypothesis of memory reconsolidation suggests that it may be possible to target the trauma memory directly, with the promise of an instantaneous and more persistent alleviation of symptoms. Memory reconsolidation refers to the process that upon memory retrieval, items in long-term memory may temporarily return into a labile state requiring de novo protein synthesis in order to persist ( 40 ). This cascade of neurobiological processes offers a window of opportunity for targeting fear memories with amnestic agents. The crucial role of central noradrenergic signaling in the process cascade ( 41 ) suggests that the β-adrenergic blocker propranolol is a viable option for effectively interfering with memory reconsolidation in humans. Indeed, preclinical research has compellingly shown that β-adrenergic blockade during reconsolidation can disrupt fear memories in healthy individuals (e.g., ( 42 ); see for a review ( 43 )) and in people with a fear of spiders (( 44 , 45 ); but (see 46 )). Even though these findings point to a revolutionary new treatment for emotional memory disorders, the success of reconsolidation interventions is not guaranteed ( 47 ). The effect of the intervention depends on whether memory retrieval effectively triggers reconsolidation. Only if the retrieval experience contains novel or unexpected information (i.e., prediction error), the memory engram will be destabilized ( 48 , 49 ). While clinical research in patients with PTSD initially revealed a reduction in fear responding following a reconsolidation intervention ( 17 , 50 – 52 ), these findings could not always be replicated in several follow-up trials (( 53 ); see for a review ( 54 )). It is worth highlighting that the design of these previous clinical trials raises several questions with respect to the necessary conditions for a reconsolidation intervention. The effectiveness of this intervention hinges on two key conditions: (i) the retrieval procedure should lead to the destabilization of the trauma memory, and (ii) the amnestic drug should interfere with the subsequent reconsolidation of that fear memory ( 43 ). In previous clinical trials, script-driven imagery was employed to reactivate traumatic memories, despite the fact that this method was explicitly designed to assess the passive retrieval of these memories, rather than their reconsolidation. Additionally, the timing of drug administration in these clinical trials (specifically, the use of long-acting propranolol after the reactivation of the traumatic memory) does not align with the reconsolidation hypothesis. We carried out a series of experiments aimed at exploring the optimal timing for administering propranolol. Our findings revealed a rather narrow temporal window, spanning less than four hours following memory reactivation, during which the β-adrenergic receptors assume a pivotal role in the reconsolidation of fear memories ( 47 , 55 ).In summary, unlike traditional trauma-focused interventions that require multiple sessions with gradual and often temporary improvements, the memory reconsolidation intervention (i.e., Memrec) offers an unique approach to treating combat-related PTSD by providing a single, possibly effective treatment session that results in a sudden reduction in emotional symptoms. This innovative treatment approach also represents a departure from the conventional use of pharmaceutical agents to alleviate PTSD symptoms, as it involves a one-time administration of a very common drug (i.e., 40 mg propranolol HCl) after reliving and rescripting the combat-related trauma memory. In the current case series involving seven veterans we tested the effectiveness of Memrec for trauma in which we aimed to address the unparalleled complexities that are typically associated with treating combat-related PTSD.
2. Materials and methods
2.1. participants.
Participants were combat-exposed Dutch military veterans referred for treatment at ARQ Centrum ‘45, the Dutch national center for diagnostics and treatment of patients with long-lasting trauma-related disorders. Inclusion criteria were (a) aged between 18–65 years, and (b) a diagnosis of PTSD based on the Clinician-Administered PTSD Scale (CAPS-5) ( 56 ). The exclusion criteria included (a) any other relevant treatment for PTSD within 3 months before the start of the study, (b) start of new psychotropic medication within 3 months before the start of the study – medication used for longer periods could be continued, (c) life-time psychosis, (d) acute suicide risk, and (e) any contra-indications for the use of propranolol. All participants gave written informed consent, and the protocol was approved by the Medical-Ethical Committee of the Amsterdam UMC.
2.2. Outcome measures
The following measures were completed pre-treatment, as well as 1-month and 3-months post-intervention.
2.2.1. PTSD checklist for DSM-5
The PCL-5 is a 20-item self-report measures that assesses the 20 DSM-5 symptoms of PTSD ( 57 ), and can be used to monitor symptom change, to screen for PTSD, or to make a provisional PTSD diagnosis. Respondents rate each item from 0 = “not at all” to 4 = “extremely” to indicate the degree to which they have been bothered by that particular symptom over the past month. A total symptom severity score can be obtained by summing the scores for each of the 20 items, range = 0–80. DSM-5 symptom cluster severity scores can be obtained by summing the scores for the items within a given cluster, i.e., cluster B = items 1–5, cluster C = items 6–7, cluster D = items 8–14, and cluster E = items 15–20. A PCL-5 cut-off score between 31–33 is considered indicative of PTSD ( 58 ). Evidence suggests that a 15–20 point change represents a clinically significant change ( 59 ). The PCL-5 is a psychometrically sound instrument that can be used effectively with veterans ( 58 , 60 ). An additional item was added to the PCL-5 to ask about distress and interference caused by PTSD symptoms on a 5-point scale of frequency and severity ranging from 0 = “not at all” to 4 = “6 or more times a week” ( 61 ).
2.2.2. Beck Depression Inventory - Second Edition
The BDI-II is a widely used 21-item self-report inventory measuring the severity of depression over the previous 2 weeks ( 62 ). The items are rated on a 4-point severity scale and are summed to give a total score, with a range of 0–63. A higher score on the BDI-II denotes more severe depression, with norms of 0–13 = minimal depression, 14–19 = mild depression, 20–28 = moderate depression, and 29–63 = severe depression. The BDI-II is considered a valid and reliable instrument ( 63 ), and is often used as outcome instrument in treatment research.
2.2.3. Mental Health Quality of Life Questionnaire
The MHQoL is a self-report questionnaire that captures and values 7 dimensions relevant to the quality of life of people with mental health problems: i.e. self-image, independence, mood, relationships, daily activities, physical health, and future. The MHQoL comprises seven questions each with four response levels, ranging from “very satisfied” to “very dissatisfied.” The MHQoL index score can vary from 0 to 21, with higher scores indicating better quality of life. The MHQoL demonstrates favorable psychometric properties, and shows promise as a simple and effective measure to assess quality of life in people with mental health problems ( 64 ).
2.3. General treatment procedure
The general procedure of the Memrec-intervention for veterans with PTSD involved a screening session, an intake session, one or two intervention sessions, and a series of three assessment sessions. During screening, the study procedures were explained, patients provided written informed consent, and the baseline assessment was completed – t0, see below. Next, a medical screening was conducted to rule out any possible contraindications for the use of propranolol, including blood pressure < 90/60 mmHg, heart rate < 60 bpm, a range of adverse medical conditions such as heart disease or asthma, and the use of other medications that may negatively interact with propranolol.
In the intake session, trauma memory was explored. Specifically, the patients’ most painful trauma memories were identified by using the contents of their intrusive symptoms as a guide. In the intervention session, these “hot spots” ( 13 , 16 ) subsequently served as the focus of the reactivation procedure. Also, patients’ safety behaviors were identified (e.g., distracting oneself), and they were instructed to drop these behaviors during reactivation.
Given the potential of multi-sensory input during treatment to increase engagement and support activation of traumatic memories ( 65 ), the reactivation procedure started with experiencing war in a “sensory-reality” application 2 , in which audio-visual experiences are synchronized with scent, temperature, air flow, and tremble. After the ±2-min film displaying relevant war images 3 , the patients were instructed to close their eyes and reactivation focused on their personal hotspots. As the patients revisited the most challenging aspects of the traumatic event, they were encouraged to envision how they wished they had responded during the trauma, aiming to address instances of moral injury they had experienced. A single dose of 40 mg of propranolol was administered immediately upon successful rescripting of the combat-related memory, which was evidenced by patients’ self-report and therapist observations. 4 Next, a 120-min waiting period followed to monitor the intended effects of propranolol on autonomic responding: blood pressure and heart rate were recorded at the beginning and end of the intervention-session.
A week after the intervention, trauma memory was again relived through both the sensory-reality pod and imaginal exposure. If patients reported no meaningful reduction of their PTSD symptoms and still showed high-end distress ratings during imaginal reliving, they were offered a second pill intake, and an extra post-intervention session followed a week later. This was repeated only once.
The assessment sessions took place at screening (t0), at 1-month (t1) and 3-months (t2) post-treatment. Assessments included the PCL-5 ( 57 ) as the primary outcome, and the BDI-II ( 62 ), and MHQoL ( 64 ) as secondary outcomes. Moreover, at these timepoints, patients were interviewed about any changes brought about by the Memrec-intervention.
Here, we present seven cases from an open-label pilot study of the reconsolidation intervention for veterans with PTSD. The patients were on the waiting list for trauma-focused treatment at ARQ Centrum ‘45, when offered the possibility of this intervention. All cases were treated by the first author (MK), who is a registered healthcare psychologist and an experienced cognitive behavioral therapist.
3.1. Case descriptions
3.1.1. case conceptualization_1.
Case 1 concerns a 47-year old veteran who had been deployed to former Yugoslavia in 1996. His PTSD symptoms developed progressively since 2001, after watching a documentary on the fall of Srebrenica. His most prominent symptoms included flashbacks alternating with amnesia of a traumatic night during the time of deployment, and being hyper-alert in public places. He had received three sessions of EMDR, without any symptom relief.
The treatment focused on that specific night, which was pitch-dark and full of explosions. While lying on his stomach in the dirt, threats were made over the radio: “fuck you IFOR, we are gonna get you.” He experienced intense fear, and eventually blacked-out: he cannot remember part of the night and is afraid he emptied his weapon, which will not let him go. With the imagery rescripting, we aimed at enabling him to tolerate the worst-case scenario: i.e. him shooting his gun. Even though overwhelmed by this, it unexpectedly felt as a relief to be able to do what he feared most had happened. He experienced intense emotions, and at several moments he cried. 40 mg of propranolol was given after reactivation.
A week later, re-experiencing the traumatic night no longer triggered any fear or anxiety, and he had stopped worrying about what might have had happened. After a month, he hardly ever thought about the particular night anymore, and his nightmares had disappeared. He felt less alert, and was now able to go to grocery stores without scanning for potential danger. His improvements remained stable over time, as reflected in (i) a drop of >30 points on the PCL-5 and (ii) his scores on the secondary measures. He no longer met the criteria for a PTSD diagnosis – see Figure 1A and Table 1 .
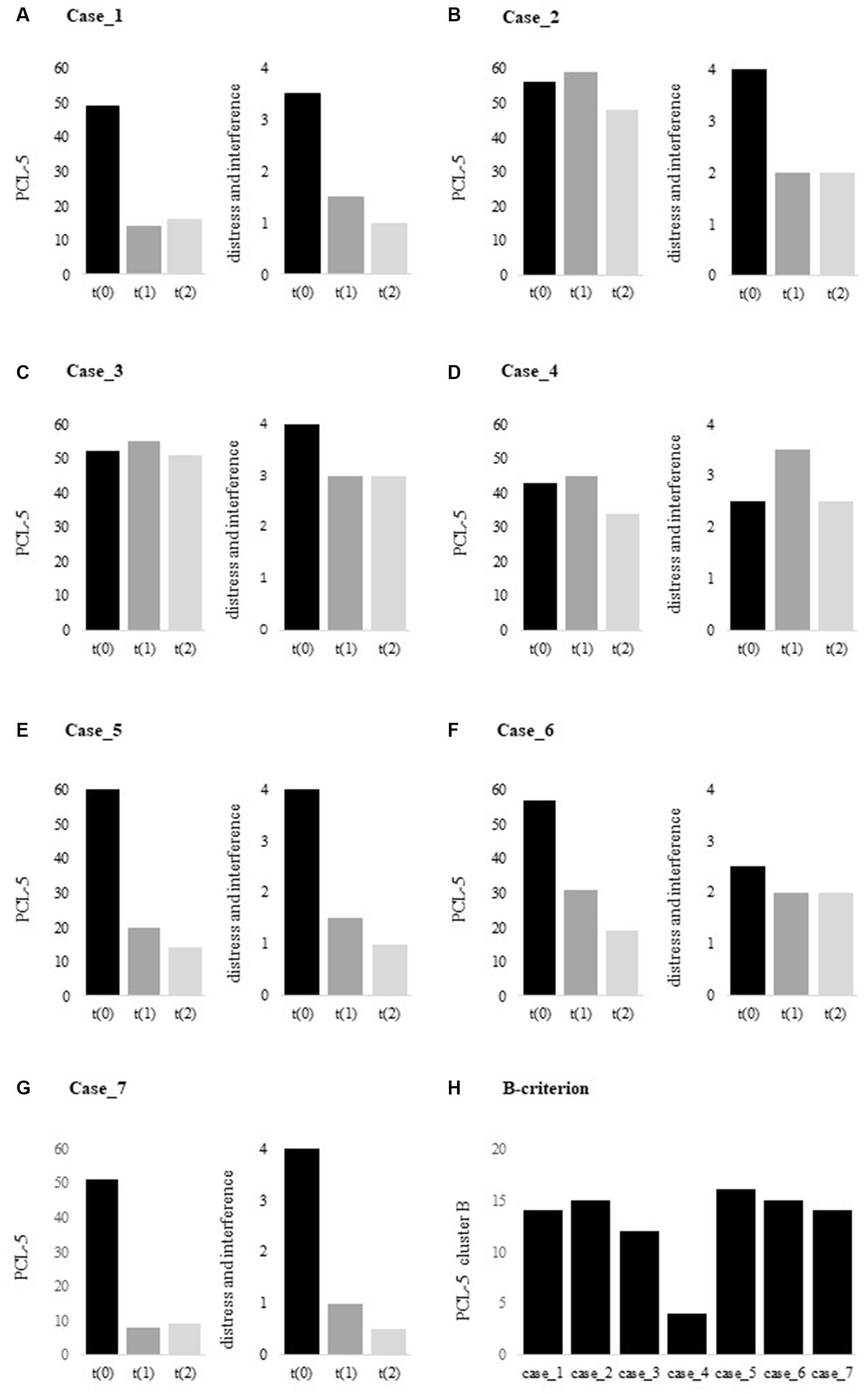
Figure 1 . (A–G) . PCL-5 total scores at t(0), t(1) and t(2), and (H) . PCL-5 cluster B scores at t(0). PCL-5, PTSD Checklist for DSM-5 [range 0–80].
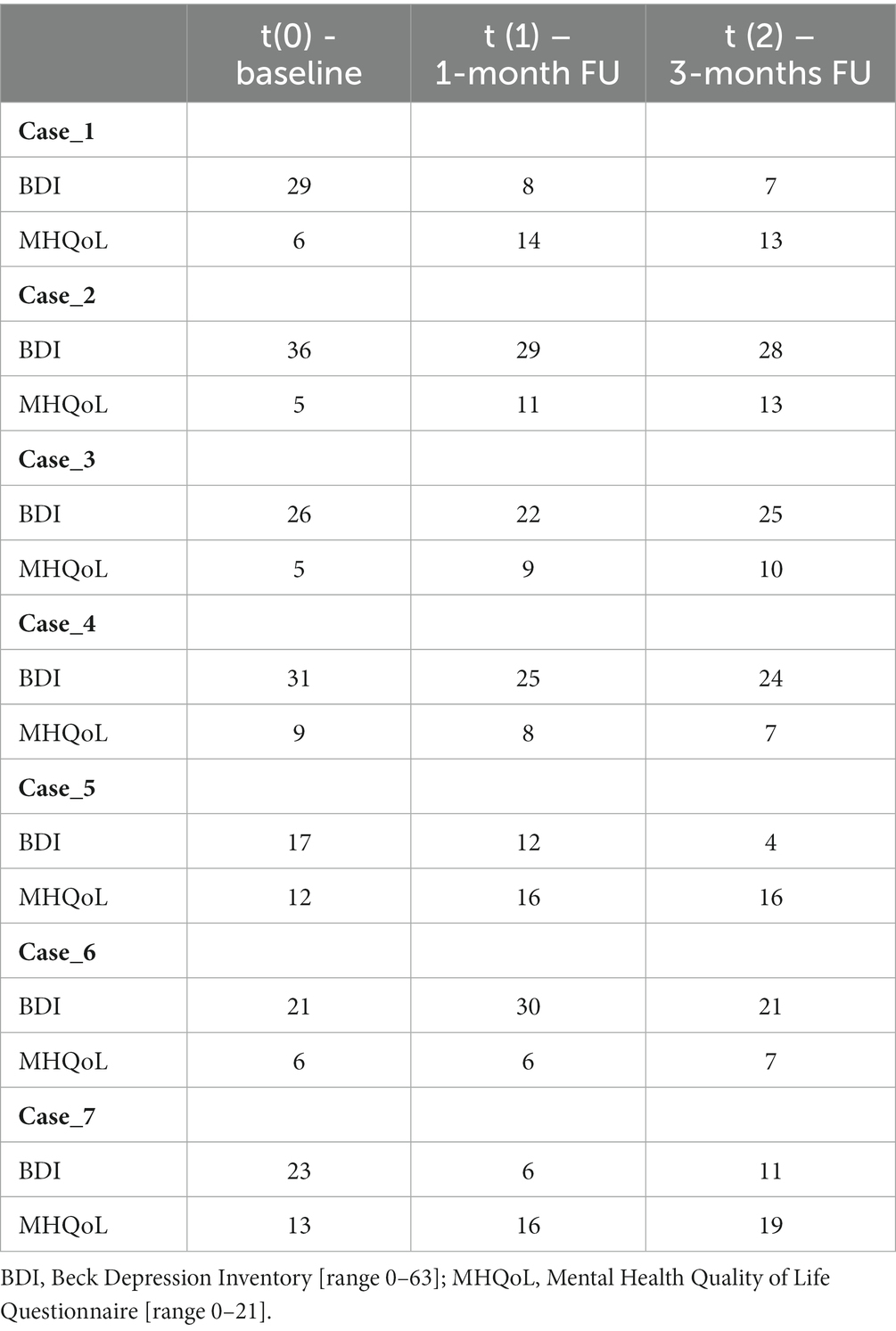
Table 1 . Patients’ total scores on the BDI and MHQoL at t(0), t (1) and t (2).
3.1.2. Case conceptualization_2
Case 2 is a 39-year old veteran who served in Afghanistan. He suffered from severe PTSD, with nightmares, flashbacks, feeling hyper-alert, and panic attacks in public places. He previously received pharmacological treatment for depression, with sufficient symptom relief. Ultra-long distance trail running offered him an outlet for releasing some of his tension and anxiety.
Here, the treatment focused on two intrusive memories where he had felt powerless: (i) a suicide attack on a market with many civilian casualties where he was unable to help, and (ii) the moment a Dutch army vehicle was struck by a roadside bomb and he was not allowed to fight. With the imagery rescripting, we aimed at inducing a feeling of being in control, i.e., contrary to what he had actually experienced. He was instructed to relive the moments and imagine his desired response, which triggered intense emotional feelings. Afterwards, 40 mg of propranolol was administered.
From the following day onwards, he experienced a breakthrough in emotions. He noticed that it was easier to talk about his traumatic experiences and to share his feelings. He constantly started challenging himself by going to public places such as grocery stores. Although this felt as a victory to him, it was also very distressing, which may be reflected in the PCL-5: still high overall scores at t5 but less interference in everyday life – see Figure 1B and Table 1 . Indeed, he experienced an improvement in quality of life and reported being optimistic about the future, where he wants to help other veterans with PTSD through organizing run clinics and events.
3.1.3. Case conceptualization_3
Case 3 concerns a 37-year old veteran who had been deployed to Iraq and Afghanistan between 2002–2011. His PTSD symptoms had developed over several years, including severe irritability or aggressive behavior, disturbed sleep, nightmares, flashbacks, and avoiding distressing memories, thoughts and feelings associated with his missions. He was diagnosed with ADHD as an adult, for which no medication. After a period of misuse of anabolic steroids in the past, he had started weekly injections of testosterone years ago to replace what his body no longer produced. He had received several non-trauma focused treatments as well as one session of EMDR, without sufficient symptom relief.
Given his diverse traumatic experiences, this treatment included two sessions followed by the intake of a pill of 40 mg propranolol. In the first session, imagery rescripting focused on an armed home invasion in Iraq, involving a playing child. We aimed at targeting his feelings of guilt by saving the child in the pertinent situation, instead of leaving her behind. During the second intervention-session, he relived the moment he was forced to leave Afghanistan due to an injury. Here we aimed at targeting his feelings of inadequacy of leaving behind his fellow soldiers as a result of his physical injuries.Throughout both interventions-sessions, he experienced intense emotions that previously he had been able to suppress.
After the Memrec-sessions, he reported to have experienced a breakthrough in trauma processing. He started to remember new events from his time of deployment, felt and shared repressed emotions, and noticed that it was easier to talk about his experiences. Although this was perceived as positive to him, his PTSD symptom severity score slightly increased – see Figure 1C . He still suffered from disrupted sleep, but note that this may also be a side-effect of the testosterone therapy ( 66 ). Despite all this, his aggressive outbursts had reduced to a minimum and he had opened up to life again, undertaking social activities with friends – see Table 1 .
3.1.4. Case conceptualizaiton_4
Case 4 is a 50-year old veteran who had been deployed to Bosnia and Afghanistan, and is still active in the army. He had suffered from emotional numbness since 1997, when two soldiers died under his command. He experienced profound guilt, a distorted sense of responsibility and failure. He previously received pharmacological treatment for depression, without sufficient symptom relief.
This treatment included two intervention-sessions. Despite both sessions having triggered intense emotional feelings while reliving the traumatic event(s), the intervention failed to improve the patient’s symptoms – see Figure 1D and Table 1 . Indeed, he reported no change in mood or mental state. Interestingly, inspecting the PCL-5 cluster scores revealed that he showed little combat-related intrusions – see Figure 1H : cluster B score of 4, with a range of 0–20. While PTSD is a complex disorder with a broad range of other negative emotions such as guilt or anhedonia – which in fact dominated this patient’s clinical picture. Memrec is exclusively designed to target unduly intense fear, which may explain the lack of effect for this particular case.
3.1.5. Case conceptualization_5
Case 5 concerns a 30-year old veteran who served in the Belgium army, and was deployed to Afghanistan in 2012. His PTSD symptoms had worsened since 2020, including nightmares, flashbacks, feeling hyper-alert, panic attacks in certain situations, a short temper or aggressive behavior, and emotional flattening and loss of interest. He had received 21 sessions of EMDR, without any symptom relief.
As part of the Quick Response Team, he had to guard the gate of an army base in Kabul, and to bring in the dead and wounded. With the first imagery rescripting session, we aimed to let him relive his emotions when sitting in front of the gate with a severely injured Afghan girl in his arms, who eventually died. Even though he was not able to picture the situation, some grief and anger surfaced, and he was administered a pill of 40 mg of propranolol. A week later, the second intervention-session focused on the situation he had to carry in coffins with corpses through the gate. This triggered intense emotional feelings, and all of a sudden he was able to vividly remember what the gate had looked like – an image of which he had completely forgotten. Again, 40 mg of propranolol was given afterwards.
After the second Memrec-session, the flashbacks and nightmares had disappeared. He felt less alert, but cheerful and happy – as was confirmed by his girlfriend. He no longer experienced panic attacks and reported it was much easier to hold his temper when frustrated, which made him feel like a better father to his one-year old son. He started living life again, and reintegrated into work. Even though he somewhat feared relapsing into PTSD, the effect persisted and improved even more over time – see Figure 1E ; Table 1 .
3.1.6. Case conceptualization_6
Case 6 is a 49-year old veteran who served in Bosnia at the time of the fall of Srebrenica. He had suppressed the painful memories and feelings for years, but lately the impacts of his trauma surfaced. He had become very emotional, startled easily, and experienced frequent panic attacks as well as severe nightmares every single night.He had not received prior treatment.
He reacted very strongly to the sensory-reality exposure, and throughout the intervention-session he was continuously in tears. With the imagery rescripting we aimed to target two intrusive hotspots: (i) leaving behind a Danish soldier who was shot in the head – he imagined apologizing to him, and (ii) the moment he had wanted to shoot an approaching enemy vehicle, but was stopped by a fellow soldier – which had made him full of guilt and regret. He was instructed to relive this moment and imagine following his impulse: shoot and become angry at the enemy. Afterwards, 40 mg of propranolol was administered.
A week later, he reported to have experienced a positive effect of the Memrec-session: he noticed it was easier to talk about his encounters in war, and was no longer overwhelmed by emotions. His panic had suddenly disappeared as did his nightmares: he never had a “nocturnal visit” from the Danish soldier again. He had always felt excessively guilty, but had let go of this feeling entirely. And even though the news about the war in Ukraine touched him deeply and made him feel somewhat depressed, his PTSD symptoms further improved over the 3-months follow-up period – see BDI-scores in Table 1 and Figure 1F .
3.1.7. Case conceptualization_7
Case 7 concerns a 44-year old veteran who had been deployed to Bosnia and Afghanistan as a vehicle recovery expert. His most prominent PTSD symptoms included recurrent nightmares, flashbacks, hypervigilance, severe irritability, and verbally aggressive behavior. He had received two sessions of exposure therapy, without any symptom relief.
The treatment focused on a hotspot memory of a nighttime firefight in Afghanistan, where two Dutch soldiers died of friendly fire and his sergeant froze with fear in the midst of the fight. He had no choice but to use a gun to survive, while – as a vehicle recovery operator – he was not trained for it. With the imagery rescripting we aimed to allow feelings of anger toward his sergeant for failing him, which he verbally acted out and, in turn, made him very emotional. Afterwards, he received 40 mg of propranolol.
A week later, he entered cheerfully. He reported that his nightmares disappeared, he had felt less alert, and that his once-frequent aggressive outbursts had reduced to zero, which was noticed by his wife and daughter. His remarkable progress remained steady, as reflected in (i) a drop of >40 points on the PCL-5 and (ii) his scores on the secondary measures – see Figure 1G and Table 1 . He no longer met the criteria for a PTSD diagnosis.
4. Discussion
The findings of this small-scale case series cautiously suggest that Memrec holds promise for producing notable effects in just one or two treatment sessions. Clearly, four out of the seven veterans experienced a remarkable reduction in their PTSD symptoms. As for the remaining three veterans, two displayed improvements in their daily distress levels, notwithstanding the absence of a reduction on the PTSD symptom scale. Only one veteran did not exhibit any progress in his PTSD and distress symptoms, but this veteran (i.e., case 4) primarily struggled with depression rather than combat-related intrusive memories (see Figure 1H ). Although the improvement in depression may appear less convincing based on these case series, three veterans showed a significant reduction in their depressive symptoms. Also, the quality of life saw a remarkable increase in most of the treated veterans (i.e., five out of the seven). It is important to note though that the long-term durability of these initial positive results remains uncertain, given that the last assessment occurred only three months after the treatment session. Nevertheless, the current findings demonstrate a general positive effect of this limited intervention, which is particularly noteworthy considering the complexity of symptoms experienced by this sample of veterans. In summary, there is reason to be optimistic for the treatment of combated-related PTSD, as it appears that there is much greater potential than commonly believed.
Certainly, we cannot conclusively attribute these effects to disrupting the process of memory reconsolidation (i.e., Memrec). Since we did not control for placebo or other trauma-focused treatments, nor for the well-known effects of a waiting list ( 67 ), the symptom reduction may also be due to other factors, including imagery rescripting, which is a fast-rising therapeutic technique yielding remarkably positive outcomes. Imagery rescripting is generally applied to treat symptoms associated with aversive mental images and is currently considered among the most effective treatments for patients with PTSD ( 68 – 71 ). Nevertheless, it is worth noting that the majority of these seven veterans seemed to be treatment resistant as they had already tried various trauma-focused treatments without any success, which at least suggests the presence of some specific active components in the current approach. The Memrec intervention was specifically designed to target trauma memories and it is therefore not surprising that one veteran who did not suffer from combat-related intrusions did not respond to the intervention. But also for the veterans who benefited from this intervention, it is evident that this treatment alone is not sufficient. Most participants (i.e., n = 6) were prior military service members with military-related PTSD, who had comorbid psychiatric conditions, had experienced multiple combat-related traumatic events, and had significant ongoing life stressors. They also grappled with profound relationship and familial challenges. Hence, there are so many other factors at play in post-war trauma survivors’ lives that Memrec or similar trauma-focused treatments should be offered as a module alongside existing treatment approaches.
Treating veterans with PTSD through memory-modifying approaches such as Memrec and Imagery Rescripting does, however, give rise to ethical considerations. These concerns encompass a range of potential issues, including the potential loss of autobiographical memories, the creation of false memories, and the unforeseen repercussions of modifying fear responses associated with traumatic memories ( 72 ). Our research findings indicate that the likelihood of losing explicit, declarative, or autobiographical memory through Memrec is very low. Individuals retained conscious awareness of previously acquired information even as the learned fear response was neutralized ( 20 , 43 ). In contrast, overwhelming emotional memories can impede a patient’s ability to recall and make sense of their experiences ( 13 ). Consequently, reducing the emotional intensity of strongly encoded emotional memories through reconsolidation-based methods might paradoxically facilitate controlled recollection and integration into a coherent personal narrative, as observed in some veterans in our study (i.e., cases 5 and 6). Rather than inducing amnesia, the administration of propranolol after memory reactivation appears to primarily reduce the intense emotional impact associated with traumatic memories. Similarly, imagery rescripting may help patients reinterpret troubling past events, thereby lessening the impact of distressing memories, but it does not have the ability to erase or alter the original trauma memory ( 73 ). Another ethical concern is that some degree of fear and stress may serve a beneficial role in combat situations, preventing impulsive decisions and preparing the body for action. Hence, it may not be desirable to interfere with these emotional responses for military purposes. Nevertheless, experiments conducted in our laboratory have shown that even after mitigating learned fear through reconsolidation interventions, individuals can readily reacquire the same fear response when exposed to new threats ( 74 ). This demonstrates that military personnel who have undergone a memory-modifying intervention are not exempt from developing new emotional responses when reintroduced to real-life battlefield hazards. A clear strength of this study is its use of distinctive multi-sensoric retrieval cues to access the trauma-memory. Still, the 3D films alongside the smells were far from optimal as they could be more idiosyncratic. In addition to advance these more technological aspects of the intervention, for a next step we should systematically assess and explore the stuck points and explicitly formulate hypotheses on where and how to intervene. The current study is also limited by a small sample size, the absence of a control group, the absence of CAPS-5 assessments during the follow-up, the omission of multiple baseline measurements and the omission of long-term FU evaluations, which are typically included in more rigorously controlled case-series designs. The gold standard, naturally, aims to convey that a Randomized Controlled Trial (RCT) is the epitome of establishing the effectiveness of an intervention. When it comes to clinical practice, RCTs are not particularly beneficial in assessing the progress of individual clients. However, they can indeed prove useful in indicating which alternative treatments would be a promising starting point. On the other hand, psychotherapy studies are facing additional criticism for relying primarily on the average responses of large treatment groups, disregarding the variations within individuals that can influence the outcomes. In light of this criticism, researchers are now placing greater emphasis on the significance of individual treatment responses and the mechanisms of therapeutic change as the most effective means to improve efficacy ( 75 ). Single-case designs present an effective research methodology that systematically addresses individual differences over a period of time. This design significantly diminishes the likelihood of attributing changes solely to factors unrelated to the treatment and the conclusions drawn from such a study surpasses what can be derived from uncontrolled case studies ( 76 ). In the continued advancement of interventions for combat-related trauma, it is imperative for future research to build upon controlled case series and thoroughly examine the effectiveness of the intervention in addressing the wide array of symptoms commonly experienced by veterans.

Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethical Committee Amsterdam UMC. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MK: Investigation, Writing – original draft. MS: Investigation, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the LZV research program of the Dutch Ministry of Defense, The Hague, The Netherlands and by an ERC Advanced Grant 743263 awarded to MK.
Acknowledgments
The authors gratefully acknowledge the assistance of Lucas Schalk, psychiatrist at ARQ Centrum ‘45, for the recruitment of patients.
Conflict of interest
MK is the co-founder of Kindt Clinics, an outpatient clinic for phobias and anxiety disorders that provides reconsolidation-based treatments.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^ Prolonged Exposure (PE), Cognitive Processing Therapy (CPT), Cognitive Behavior Therapy (CBT) and Eye Movement Desensitization Reprocessing (EMDR).
2. ^ https://www.sensiks.com/ . With the SENSIKS reality pod various war zones can be simulated.
3. ^ Video0 12 - YouTube for Afghanistan-deployed veterans, and sensiks forest final3 - YouTube for veterans who served in former Yugoslavia.
4. ^ See the documentary Nighttime in Kabul | A Cure For Fear: Part 2 | Topic - YouTube by Lana Wilson, in which a Canadian veteran was successfully treated using this approach.
1. Eekhout, I, Geuze, E, and Vermetten, E. The long-term burden of military deployment on the health care system. J Psychiatr Res . (2016) 79:78–85. doi: 10.1016/j.jpsychires.2016.05.004
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Reijnen, A, Rademaker, AR, Vermetten, E, and Geuze, E. Prevalence of mental health symptoms in Dutch military personnel returning from deployment to Afghanistan: a 2-year longitudinal analysis. Eur Psychiatry . (2015) 30:341–6. doi: 10.1016/j.eurpsy.2014.05.003
3. Richardson, LK, Frueh, BC, and Acierno, R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry . (2010) 44:4–19. doi: 10.3109/00048670903393597
4. Creamer, M, Wade, D, Fletcher, S, and Forbes, D. PTSD among military personnel. Int J Rev Psych . (2011) 23:160–5. doi: 10.3109/09540261.2011.559456
CrossRef Full Text | Google Scholar
5. Watts, BV, Schnurr, PP, Mayo, L, Young-Xu, Y, Weeks, WB, and Friedman, MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry . (2013) 74:e541–50. doi: 10.4088/JCP.12r08225
6. Haagen, JF, Smid, GE, Knipscheer, JW, and Kleber, RJ. Efficacy of recommended treatments for veterans with PTSD: a meta-regression analysis. Clin Psychol Rev . (2015) 40:184–94. doi: 10.1016/j.cpr.2015.06.008
7. Steenkamp, MM, Litz, BT, Hoge, CW, and Marmar, CR. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA . (2015) 314:489–500. doi: 10.1001/jama.2015.8370
8. Weber, M, Schumacher, S, Hannig, W, Barth, J, Lotzin, A, Schafer, I, et al. Long-term outcomes of psychological treatment for posttraumatic stress disorder: as systematic review and met-analysis. Psychol Med . (2021) 51:1420–30. doi: 10.1017/S003329172100163X
9. Steenkamp, MM, Litz, BT, and Marmar, CR. First-line psychotherapies for military-related PTSD. JAMA . (2020) 323:656–7. doi: 10.1001/jama.2019.20825
10. Sciarrino, NA, Bartlett, BA, Smith, LJ, Martin, CE, and Williams, W. Factors contributing to PTSD treatment dropout in veterans returning from the wars in Iraq and Afghanistan: a systemic review. Psychol Serv . (2022) 19:183–200. doi: 10.1037/ser0000519
11. Rubin, DC, Berntsen, D, and Bohni, MK. A memory-based model of posttraumatic stress disorder: evaluating basic assumptions underlying the PTSD diagnosis. Psychol Rev . (2008) 115:985–1011. doi: 10.1037/a0013397
12. Kindt, M. A behavioural neuroscience perspective on the aetiology and treatment of anxiety disorders. Beh Res Ther. (2014) 62:24–36. doi: 10.1016/j.brat.2014.08.012
13. Ehlers, A, and Clark, D. A cognitive model of post-traumatic stress disorder. Behav Res Ther . (2000) 38:319–45. doi: 10.1016/S0005-7967(99)00123-0
14. Grey, N, Holmes, E, and Brewin, CR. Peritraumatic emotional “hotspots” in memory. Beh Cog Psychotherapy . (2001) 29:367–72. doi: 10.1017/S1352465801003095
15. Grey, N, Young, K, and Holmes, E. Cognitive restructuring within reliving: a treatment for peritraumatic emotional “hotspots” in posttraumatic stress disorder. Beh Cog Psychotherapy. (2002) 30:37–56. doi: 10.1017/S1352465802001054
16. Holmes, E, Grey, N, and Young, K. Intrusive images and “hotspots” of trauma memories in post-traumatic disorder: an exploratory investigation of emotions and cognitive themes. J Behav Ther Exp Psychiatry . (2005) 36:3–17. doi: 10.1016/j.jbtep.2004.11.002
17. Kindt, M, and Emmerik, AAP. New avenues for treating emotional memory disorders: towards a reconsolidation intervention for posttraumatic stress disorder. Ther Adv Psychopharmacol . (2016) 6:283–95. doi: 10.1177/2045125316644541
18. David, D, and Szentagotai, A. Cognitions in cognitive-behavioral psychotherapies; toward an integrative model. Clin Psychol Rev . (2006) 26:284–98. doi: 10.1016/j.cpr.2005.09.003
19. Arntz, A, De Groot, C, and Kindt, M. Emotional memory is perceptual. J Beh Ther Exp Psych. (2005) 36:19–34. doi: 10.1016/j.jbtep.2004.11.003
20. Kindt, M, Buck, N, Arntz, A, and Soeter, M. Perceptual and conceptual processing as predictors of treatment outcome in PTSD. J Beh Ther Exp Psych . (2007) 38:491–506. doi: 10.1016/j.jbtep.2007.10.002
21. Levi, O, Ben Yehuda, A, Pine, DS, and Bar-Haim, Y. A sobering look at treatment effectiveness of military-related posttraumatic stress disorder. Clin Psychol Sci . (2022) 10:690–9. doi: 10.1177/21677026211051314
22. Serfioti, D, Murphy, D, Greenberg, N, and Williamson, V. Effectiveness of treatments for symptoms of post-trauma related guilt, shame and anger in military and civilian populations: a systematic review. BMJ Mil Health . (2022):e002155. doi: 10.1136/military-2022-002155
23. Williamson, V, Murphy, D, Stevelink, SA, Allen, S, Jones, E, and Greenber, N. The impact of trauma exposure and moral injury on UK military veterans: a qualitative study. Eur J Psychotraumatol . (2020) 11:1704554. doi: 10.1080/20008198.2019.1704554
24. Litz, BT, Stein, N, Delaney, E, Lebowitz, L, Nash, WP, Silva, C, et al. Moral injury and moral repair in war veterans: a preliminary model and interventions strategy. Clin Psychol Rev . (2009) 29:695–706. doi: 10.1016/j.cpr.2009.07.003
25. Griffin, BJ, Purcell, N, Burkman, K, Litz, BT, Bryan, CJ, Schmitz, M, et al. Moral injury: an integrative review. J Traum Stress . (2019) 32:350–62. doi: 10.1002/jts.22362
26. Williamson, V, Greenberg, N, and Murphy, D. Moral injury in UK armed forces veterans: a qualitative study. Eur J Psychotraumatol . (2019) 10:1562842. doi: 10.1080/20008198.2018.1562842
27. Chu, S, and Downes, JJ. Proust nose best: odors are better cues of autobiographical memory. Mem Cogn . (2002) 30:511–8. doi: 10.3758/BF03194952
28. Daniels, JK, and Vermetten, E. Odor-induced recall of emotional memories in PTSD – review and new paradigm for research. Exp Neurol . (2016) 284:168–80. doi: 10.1016/j.expneurol.2016.08.001
29. Hackländer, RP, Janssen, SM, and Bermeitinger, C. An in-depth review of the methods, findings, and theories associated with odor-evoked autobiographical memory. Psych Bull Rev . (2019) 26:401–29. doi: 10.3758/s13423-018-1545-3
30. Hakim, M, Battle, AR, Belmer, A, Bartlett, SE, Johnson, LR, and Chehrehasa, F. Pavlovian olfactory fear conditioning: its neural circuity and importance for understanding clinical fear-based disorders. Fr Mol Neurosci . (2019) 12:221. doi: 10.3389/fnmol.2019.00221
31. Herz, RS. The role of odor-evoked memory in psychological and physiological health. Brain Sci . (2016) 6:22. doi: 10.3390/brainsci6030022
32. Doty, RL. Olfaction. Ann Rev Psychol . (2001) 52:423–52. doi: 10.1146/annurev.psych.52.1.423
33. Wilson, DA, Best, AR, and Sullivan, RM. Plasticity in the olfactory system: lessons for the neurobiology of memory. Neuroscientist . (2004) 10:513–24. doi: 10.1177/1073858404267048
34. Arntz, A, Tiesema, M, and Kindt, M. Treatment of PTSD: a comparison of imaginal exposure with and without imagery rescripting. J Beh Ther Exp Psych. (2007) 38:345–70. doi: 10.1016/j.jbtep.2007.10.006
35. Arntz, A. Imagery rescripting as a therapeutic technique: review of clinical trials, basic studies, and research agenda. J Exp Psychopathol . (2012) 3:189–208. doi: 10.5127/jep.024211
36. Bouton, ME. Context, ambiquity, and unlearning: sources of relapse after behavioral extinction. Biol Psych . (2002) 52:976–86. doi: 10.1016/S0006-3223(02)01546-9
37. Nader, K. Memory traces unbound. Tr Neurosci . (2003) 26:65–72. doi: 10.1016/S0166-2236(02)00042-5
38. Hofmann, SG, and Smits, JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psych . (2008) 69:621–32. doi: 10.4088/JCP.v69n0415
39. Craske, M, Treanor, M, Conway, C, Zbozinek, T, and Vervliet, B. Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther . (2014) 58:10–23. doi: 10.1016/j.brat.2014.04.006
40. Nader, K, Schafe, GE, and LeDouw, JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature . (2000) 406:722–6. doi: 10.1038/35021052
41. Otis, JM, Werner, CT, and Mueller, D. Noradrenergic regulation of fear and drug-associated memory reconsolidation. Neuropsychopharmacology . (2015) 40:793–803. doi: 10.1038/npp.2014.243
42. Kindt, M, Soeter, M, and Vervliet, B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci . (2009) 12:256–8. doi: 10.1038/nn.2271
43. Elsey, JW, Van Ast, VA, and Kindt, M. Human memory reconsolidation: a guiding framework and critical review of the evidence. Psyhol Bull . (2018) 144:797–848. doi: 10.1037/bul0000152
44. Soeter, M, and Kindt, M. An abrupt transformation of phobic behavior after a post-retreival amnesic agent. Biol Psych. (2015) 78:880–6. doi: 10.1016/j.biopsych.2015.04.006
45. Filmer, AI, Peters, J, Bridge, LA, Visser, RM, and Kindt, M. Over the edge: extending the duration of a reconsolidation intervention for spider fear. Trans Psych. (2022) 12:261. doi: 10.1038/s41398-022-02020-x
46. Elsey, JW, and Kindt, M. Placebo and non-specific effects in reconsolidation-based treatment for arachnophobia. Fr Psych . (2021) 12:775770. doi: 10.3389/fpsyt.2021.775770
47. Kindt, M. The surprising subtleties of changing fear memory: a challenge for translational science. Phil Trnas R Soc B: Biol Sci . (2018) 373:20170033. doi: 10.1098/rstb.2017.0033
48. Sevenster, D, Beckers, T, and Kindt, M. Prediction error governs pharmacologically induced amnesia for learned fear. Science . (2013) 339:830–3. doi: 10.1126/science.1231357
49. Merlo, E, Milton, AL, Goozée, ZY, Theobald, DE, and Everitt, BJ. Reconsolidation and extinctin are dissociable and mutually exclusive processes: behavioral and molecular evidence. J Neurosci . (2014) 34:2422–31. doi: 10.1523/JNEUROSCI.4001-13.2014
50. Brunet, A, Orr, SP, Tremblay, J, Robertson, K, Nader, K, and Pitman, RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psych Res . (2008) 42:503–6. doi: 10.1016/j.jpsychires.2007.05.006
51. Brunet, A, Poundja, J, Tremblay, J, Bui, E, Thomas, E, Orr, SP, et al. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol . (2011) 31:547–50. doi: 10.1097/JCP.0b013e318222f360
52. Poundja, J, Sance, S, Tremblay, J, and Brunet, A. Trauma reactivation under the influence of propranolol: an examination of clinical predictors. Eur J Psychotraumatol . (2012) 3:15470. doi: 10.3402/ejpt.v3i0.15470
53. Wood, NE, Rosasco, ML, Suris, AM, Spring, JD, Marin, MF, Lasko, NB, et al. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psych Res . (2015) 225:31–9. doi: 10.1016/j.psychres.2014.09.005
54. Astill Wright, L, Horstmann, L, Holmes, EA, and Bisson, JI. Consolidation/reconsolidation therapies for the prevention and treatment of PTSD and re-experiencing: a systematic review and meta-analysis. Trans Psych . (2021) 11:453. doi: 10.1038/s41398-021-01570-w
55. Kindt, M, and Soeter, M. Pharmacologically induced amnesia for learned fear is time and sleep dependent. Nat Commun . (2018) 9:1316. doi: 10.1038/s41467-018-03659-1
56. Boeschoten, MA, Aa van der, N, Bakker, A, Ter Heide, FJJ, Hoofwijk, MC, Jongedijk, RA, et al. Development and evaluation of the Dutch clinician-administered PTSD scale for DSM-5 (CAPS-5). Eur J Psychotraumatol . (2018) 9:1546085. doi: 10.1080/20008198.2018.1546085
57. Weathers, FW, Litz, BT, Keane, TM, Palmieri, PA, Marx, BP, and Schnurr, PP (2013). The PTSD Checklist for DSM-5 (PCL-5) – Standard [Measurement Instrument] . Available at: https://www.ptsd.va.gov/ .
Google Scholar
58. Bovin, MJ, Marx, BP, Weathers, FW, Gallagher, MW, Rodriguez, P, Schnurr, PP, et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess . (2016) 28:1379–91. doi: 10.1037/pas0000254
59. Marx, BP, Lee, DJ, Norman, SB, Bovin, MJ, Sloan, DM, Weathers, FW, et al. Reliable and clinically significant change in the clinician-administered PTSD scale for DSM-5 and PTSD checklist for DSM-5 among male veterans. Psychol Assess . (2022) 34:197–203. doi: 10.1037/pas0001098
60. Wortmann, JH, Jordan, AH, Weathers, FW, Resick, PA, Dondanville, KA, Hall-Clark, B, et al. Psychometric analysis of the PTSD Checklist-5 (PCL-) among treatment-seeking military service members. Psychol Assess . (2016) 28:1392–403. doi: 10.1037/pas0000260
61. Foa, EB, Riggs, DS, Dancu, CV, and Rothbaum, BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Traum Stress. (1993) 6:459–73. doi: 10.1002/jts.2490060405
62. Beck, A, Steer, RA, and Brown, GK. Beck depression inventory – Second edition: Manual . San Antonio, TX: The Psychological Corporation (1996).
63. Vanheule, S, Desmet, M, Groenvynck, H, Rosseel, Y, and Fotaine, J. The factor structure of the Beck depression inventory-II: an evaluation. Assessment . (2008) 15:177–87. doi: 10.1177/1073191107311261
64. Krugten, FCW, Busschbach, JJV, Versteegh, MH, Hakkaart-van Roijen, L, and Brouwer, WBF. The mental health quality of life questionnaire (MHQoL): development and first psychometric evaluation of a new measure to assess quality of life in people with mental health problems. Qual Life Res . (2021) 31:633–43. doi: 10.1007/s11136-021-02935-w
65. Gelderen, MJ, Nijdam, MJ, and Vermetten, E. An innovative framework for delivering psychotherapy to patients with treatment-resistant posttraumatic stress disorder: rationale for interactive motion-assisted therapy. Front Psych . (2018) 9:176. doi: 10.3389/fpsyt.2018.00176
66. Liu, PY, Yee, B, Wishart, SM, Jimenez, M, Gun Jung, D, Grunstein, RR, et al. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endo Meta . (2003) 88:3605–13. doi: 10.1210/jc.2003-030236
67. Arrindell, WA. Changes in waiting-list patients over time: data on some commonly-used measures beware! Beh Res Ther . (2001) 39:1227–47. doi: 10.1016/S0005-7967(00)00104-2
68. Müller-Engelmann, M, and Steil, R. Cognitive restructuring and imagery modification for posttraumatic stress disorder (CRIM-PTSD): a pilot study. J Beh Ther Exp Psych. (2017) 54:44–50. doi: 10.1016/j.jbtep.2016.06.004
69. Romano, M, Moscovitch, DA, Huppert, JD, Reimer, SG, and Moscovitch, M. The effecs of imagery rescripting on memory outcomes in social anxiety disorder. J Anx Dis . (2020) 69:102169. doi: 10.1016/j.janxdis.2019.102169
70. Nilsson, JE, Knutsson, J, Jalamo, BS, and Lundh, LG. Imagery rescripting of early memories in health anxiety disorder: a feasibility and non-randomized pilot study. J Beh Ther Exp Psych. (2019) 65:101491. doi: 10.1016/j.jbtep.2019.101491
71. Schäfer, I, Gast, U, Hormann, A, Knaevelsrud, C, Lampe, A, Liebermann, P, et al. S3-Leitlinie Posttraumatische Belastungsstöring . Berlin, Germany: Springer (2019).
72. Elsey, JW, and Kindt, M. Manipulating human memory through reconsolidation: ethical implications of a new therapeutic approach. AJOB Neurosci . (2016) 7:225–36. doi: 10.1080/21507740.2016.1218377
73. Mortiz, S, Ahlf-Schumacher, J, Hottenrott, B, Peter, U, Franck, S, Schnell, T, et al. We cannot change the past, but we can change its meaning. A randomized controlled trial on the effects of self-help imagery rescripting on depression. Beh Res Ther. (2018) 104:74–83. doi: 10.1016/j.brat.2018.02.007
74. Soeter, M, and Kindt, M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn Mem . (2011) 18:357–66. doi: 10.1101/lm.2148511
75. Barlow, DH, Bullis, JR, Comer, JS, and Ametaj, AA. Evidence-based psychological treatments: an update and a way forward. Ann Rev Clin Psychol . (2013) 9:1–27. doi: 10.1146/annurev-clinpsy-050212-185629
76. Kazdin, AE. Single-case experimental designs. Evaluating interventions in research and clinical practice. Beh Res Ther. (2019) 117:3–17. doi: 10.1016/j.brat.2018.11.015
Keywords: reconsolidation, imagery rescripting, trauma memory, treatment, veterans, PTSD
Citation: Kindt M and Soeter M (2023) A brief treatment for veterans with PTSD: an open-label case-series study. Front. Psychiatry . 14:1260175. doi: 10.3389/fpsyt.2023.1260175
Received: 17 July 2023; Accepted: 10 October 2023; Published: 19 October 2023.
Reviewed by:
Copyright © 2023 Kindt and Soeter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Merel Kindt, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Cognitive-Behavioral Treatment of PTSD With a Young Boy and His Mother Following the Experience of Chronic Domestic Violence
- Clinical Case Studies 17(3):166-187
- 17(3):166-187

- Yale University

- University of Central Florida
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Clin Case Stud

- Jessie Faber

- Marta L. Cruz

- Robert S. Marvin

- Frank W. Putnam

- David Wechsler
- J FAM PSYCHOL

- Amanuel Medhanie
- Sara S. Sparrow

- Blanche Freund

- A.E. Schweder
- CHILD ADOL PSYCH CL
- Judith A. Cohen
- Anthony P. Mannarino
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
A Review on Post-traumatic Stress Disorder (PTSD): Symptoms, Therapies and Recent Case Studies
Affiliations.
- 1 School of Pharmacy, College of Pharmacy, Taipei Medical University, 250 Wuxing Street, Taipei 11031, Taiwan.
- 2 School of Pharmacy, Abhilashi University, Chail Chowk, tehsil Chachyot, Mandi, Himachal Pradesh 175028, India.
- 3 Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Ghal Kalan, G.T Road, Moga, Punjab, 142001, India.
- PMID: 34036925
- DOI: 10.2174/1874467214666210525160944
Post-traumatic stress disorder (PTSD), previously known as battle fatigue syndrome or shell shock, is a severe mental disturbance condition that is normally triggered by the experience of some frightening/scary events or trauma where a person undergoes some serious physical or mental harm or threatened. PTSD is a long-life effect of the continuous occurrence of traumatic conditions, leading to the production of feelings of helplessness, intense fear, and horror in the person. There are various examples of events that can cause PTSD, such as physical, mental, or sexual assault at home or working place by others, unexpected death of a loved one, an accidental event, war, or some kind of natural disaster. Treatment of PTSD includes the removal or reduction of these emotional feelings or symptoms with the aim to improve the daily life functioning of a person. Problems which are needed to be considered in case of PTSD like ongoing trauma, abusive or bad relationships. Various drugs which are used for the treatment of PTSD include selective serotonin reuptake inhibitors (SSRIs) (citalopram, fluvoxamine, fluoxetine, etc.); tricyclic antidepressants (amitriptyline and isocarboxazid); mood stabilizers (Divalproex and lamotrigine); atypical antipsychotics (aripiprazole and quetiapine), etc. In this review, we have covered the different risk factors, case studies related to various treatment options with different age group of peoples with PTSD and their effects on them. We have also covered the symptoms and associated disorders which can play a key role in the development of PTSD.
Keywords: CBT; Post-traumatic stress disorder (PTSD); SSRIs; acute stress disorder; case studies; risk factors.
Copyright© Bentham Science Publishers; For any queries, please email at [email protected].
PubMed Disclaimer
Similar articles
- [Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context]. Auxéméry Y. Auxéméry Y. Encephale. 2012 Oct;38(5):373-80. doi: 10.1016/j.encep.2011.12.003. Epub 2012 Jan 24. Encephale. 2012. PMID: 23062450 Review. French.
- Nabilone for the Treatment of Post-Traumatic Stress Disorder: A Review of Clinical Effectiveness and Guidelines [Internet]. Cowling T, MacDougall D. Cowling T, et al. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019 Feb 20. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019 Feb 20. PMID: 31553551 Free Books & Documents. Review.
- [Post-traumatic stress, post-traumatic depression and major depressive episode: literature]. Ducrocq F, Vaiva G, Cottencin O, Molenda S, Bailly D. Ducrocq F, et al. Encephale. 2001 Mar-Apr;27(2):159-68. Encephale. 2001. PMID: 11407268 Review. French.
- Post-traumatic Stress Disorder. Javidi H, Yadollahie M. Javidi H, et al. Int J Occup Environ Med. 2012 Jan;3(1):2-9. Int J Occup Environ Med. 2012. PMID: 23022845 Review.
- [Post-traumatic stress disorder in reaction to psychotic experience: A systematic revue]. Galliot G, Very E, Schmitt L, Rouch V, Salles J. Galliot G, et al. Encephale. 2019 Dec;45(6):506-512. doi: 10.1016/j.encep.2019.07.006. Epub 2019 Sep 11. Encephale. 2019. PMID: 31521338 French.
- Inhibition of Protein Synthesis Attenuates Formation of Traumatic Memory and Normalizes Fear-Induced c-Fos Expression in a Mouse Model of Posttraumatic Stress Disorder. Zamorina TA, Ivashkina OI, Toropova KA, Anokhin KV. Zamorina TA, et al. Int J Mol Sci. 2024 Jun 13;25(12):6544. doi: 10.3390/ijms25126544. Int J Mol Sci. 2024. PMID: 38928250 Free PMC article.
- Effects of mindfulness-based cognitive therapy for Chinese adults with PTSD symptoms: protocol for a randomised controlled trial. Mak BSW, Zhang D, Powell CLYM, Leung MKW, Lo HHM, Yang X, Yip BHK, Lee EKP, Xu Z, Wong SYS. Mak BSW, et al. BMC Psychiatry. 2024 May 29;24(1):400. doi: 10.1186/s12888-024-05840-x. BMC Psychiatry. 2024. PMID: 38812001 Free PMC article. Clinical Trial.
- Is post-traumatic stress disorder related to the severity of physical trauma? Aydogdu HI, Koca Y, Cirakoglu E, Anolay NN. Aydogdu HI, et al. Rev Assoc Med Bras (1992). 2023 Sep 18;69(9):e20230439. doi: 10.1590/1806-9282.20230439. eCollection 2023. Rev Assoc Med Bras (1992). 2023. PMID: 37729370 Free PMC article.
- "Mens Sana in Cute Sana"-A State of the Art of Mutual Etiopathogenetic Influence and Relevant Pathophysiological Pathways between Skin and Mental Disorders: An Integrated Approach to Contemporary Psychopathological Scenarios. Papa V, Li Pomi F, Borgia F, Genovese S, Pioggia G, Gangemi S. Papa V, et al. Cells. 2023 Jul 12;12(14):1828. doi: 10.3390/cells12141828. Cells. 2023. PMID: 37508493 Free PMC article. Review.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Bentham Science Publishers Ltd.
- Ingenta plc
Other Literature Sources
- scite Smart Citations
- Genetic Alliance
- MedlinePlus Health Information
Research Materials
- NCI CPTC Antibody Characterization Program
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Posttraumatic Stress Disorder (PTSD)
What is ptsd.
You may have posttraumatic stress disorder (PTSD), if you’ve been through a traumatic event and are having trouble dealing with it. Such events may include a car crash, rape, domestic violence, military combat, or violent crime. While it is normal to have some anxiety after such an event, it usually goes away in time. But with PTSD, the anxiety is more intense and keeps coming back. And the trauma is relived through nightmares, intrusive memories, and flashbacks. These can be vivid memories that seem real. The symptoms of PTSD can cause problems with relationships and make it hard to cope with daily life. But it can be treated. With help, you can feel better.
What causes PTSD?
PTSD may be triggered by something that:
- Happened to you
- Happened to someone close to you
- You witnessed
Examples include:
- Serious accidents, such as car or train wrecks
- Natural disasters, such as floods or earthquakes
- Manmade tragedies, such as bombings, a plane crash, shooting
- Violent personal attacks, such as a mugging, rape, torture, being held captive, or kidnapping
- Military combat
- Abuse in childhood
What are the risk factors for PTSD?
There are many risk factors for developing PTSD. Recognizing and addressing them can help prevent PTSD, when possible. These risk factors include:
- Lack of family or social support resources
- Repeated exposure to traumatic circumstances
- Personal history of trauma or of an acute stress or anxiety disorder
- Family history of mental health disorders
- Personality traits of vulnerability and a lack of resilience
- History of childhood trauma
- Personality disorder or traits including borderline personality disorder, paranoia, dependency, or antisocial tendencies
What are the symptoms of PTSD?
Symptoms of PTSD last more than a month. They may include:
- Unwanted or intense memories of a trauma
- Vivid memories or flashbacks that make you feel like you’re reliving the event
- Feeling worried, fearful, anxious, or suspicious
- Strong reactions when you’re reminded of the trauma (or sometimes for no obvious reason at all)
- Intrusive thoughts about combat, death, or killing
- Feeling disconnected or isolated, as if you’re “not yourself”
- Loss of interest in things you once enjoyed
- Feeling agitated, tense, on edge, or easily startled
- Bursts of anger or irritation
- Problems concentrating
- Trouble falling or staying asleep
The symptoms of PTSD may look like other mental health conditions. Always consult your health care provider for a diagnosis.
How is PTSD diagnosed?
Not every person who goes through a trauma develops PTSD, or experiences symptoms at all. PTSD is diagnosed if your symptoms last more than one month. Symptoms usually begin within 3 months of the trauma, but can also start months or years later.
How long this illness lasts varies. Some people recover within 6 months, others have symptoms that last much longer.
How is PTSD treated?
Specific treatment for PTSD will be decided by your healthcare provider based on:
- Your age, overall health, and medical history
- Extent of the disease
- Your tolerance for specific medicines, procedures, or therapies
- Expectations for the course of the disease
- Your opinion or preference
You may think that asking for help is a sign of weakness. In fact, taking action to make your life better takes a lot of courage. Talking about a trauma can be hard, but it can make a big difference. The main treatment for PTSD is counseling. You’ll work with a trained therapist to learn new ways to cope with your experiences. Medicine may also be prescribed to help with anxiety, depression, or sleep. Most people with PTSD have a combination of counseling and medicine for treatment.
Types of Counseling
Counseling is done in a safe environment, either one-on-one or in a group. Group therapy is often done with other people who have been through similar events. PTSD is often treated with one or more of the following forms of counseling. Talk with your healthcare provider about your options so you can decide on a counseling format that works for you.
- Cognitive processing therapy. This type of therapy helps you cope with negative thoughts related to the trauma. You’ll work with a therapist to better understand how you think and feel about what happened. And you’ll learn skills to help you cope with the trauma. CPT won’t make you forget about what happened. But it can make the memories easier to live with.
- Prolonged exposure therapy. This helps you deal with thoughts and situations related to the trauma in new ways. You’ll learn breathing and relaxation techniques to calm yourself when you encounter triggers. With your therapist’s help, you may enter situations that remind you of the trauma. You’ll learn to lessen your reactions over time, which can help with avoidance. You’ll also talk about the trauma to help you gain control over how you think and feel about it.
- Other therapies. Other therapies for PTSD include: coping skills training, acceptance and commitment training, eye movement desensitization and reprocessing (EMDR), family counseling, and PTSD psychoeducation.
- PTSD is a mental health condition in which a person has experienced a traumatic event that causes long-term stress.
- The traumatic event can be experienced directly, witnessed, or due to repeated exposure to shocking events. A person can also have PTSD when trauma occurs to a close friend or family member.
- The person may experience flashbacks, avoid stressful situations, or withdraw emotionally.
- Diagnosis is made by a healthcare provider when the symptoms last longer than one month.
- Treatment involves medicine and therapy to decrease the emotional effects of the disorder and increase coping skills.
Tips to help you get the most from a visit to your healthcare provider:
- Know the reason for your visit and what you want to happen.
- Before your visit, write down questions you want answered.
- Bring someone with you to help you ask questions and remember what your healthcare provider tells you.
- At the visit, write down the name of a new diagnosis, and any new medicines, treatments, or tests. Also write down any new instructions your healthcare provider gives you.
- Know why a new medicine or treatment is prescribed, and how it will help you. Also know what the side effects are.
- Ask if your condition can be treated in other ways.
- Know why a test or procedure is recommended and what the results could mean.
- Know what to expect if you do not take the medicine or have the test or procedure.
- If you have a follow-up appointment, write down the date, time, and purpose for that visit.
- Know how you can contact your healthcare provider if you have questions.
Find a Doctor
Specializing In:
- Deconditioning
- Child Development and Behavioral Health
- Child and Adolescent Psychiatry
- Anxiety Disorders
Find a Treatment Center
- Child and Adolescent Psychiatry (Johns Hopkins Children's Center)
- Anxiety Disorders Clinic at Johns Hopkins Bayview
Find Additional Treatment Centers at:
- Howard County Medical Center
- Sibley Memorial Hospital
- Suburban Hospital

Request an Appointment

Panic Disorder

Anxiety and Heart Disease
Related Topics
- Mental and Behavioral Health
- DOI: 10.1177/15346501241271982
- Corpus ID: 271666154
Integrating brief exposure exercises to support cognitive processing therapy for treatment of PTSD: A case study
- Alyssa M Medenblik , Todd M. Moore , Gregory L Stuart
- Published in Clinical Case Studies 1 August 2024
- Psychology, Medicine
19 References
The use of both prolonged exposure and cognitive processing therapy in the treatment of a person with ptsd, multiple traumas, depression, and suicidality, comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among us veterans a randomized clinical trial, resolution of trauma-related guilt following treatment of ptsd in female rape victims: a result of cognitive processing therapy targeting comorbid depression, the effect of cognitive processing therapy on cognitions: impact statement coding., psychometric properties of the ptsd checklist for diagnostic and statistical manual of mental disorders-fifth edition (pcl-5) in veterans., concordance in ptsd symptom change between dsm-5 versions of the clinician-administered ptsd scale (caps-5) and ptsd checklist (pcl-5)., functional analysis in differential diagnosis, the clinician-administered ptsd scale for dsm–5 (caps-5): development and initial psychometric evaluation in military veterans, the posttraumatic stress disorder checklist for dsm-5 (pcl-5): development and initial psychometric evaluation., a brief measure for assessing generalized anxiety disorder: the gad-7., related papers.
Showing 1 through 3 of 0 Related Papers
- Patient Care & Health Information
- Diseases & Conditions
- Post-traumatic stress disorder (PTSD)
Post-traumatic stress disorder (PTSD) is a mental health condition that's triggered by a terrifying event — either experiencing it or witnessing it. Symptoms may include flashbacks, nightmares and severe anxiety, as well as uncontrollable thoughts about the event.
Most people who go through traumatic events may have temporary difficulty adjusting and coping, but with time and good self-care, they usually get better. If the symptoms get worse, last for months or even years, and interfere with your day-to-day functioning, you may have PTSD.
Getting effective treatment after PTSD symptoms develop can be critical to reduce symptoms and improve function.
Products & Services
- A Book: Mayo Clinic Family Health Book
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Post-traumatic stress disorder symptoms may start within one month of a traumatic event, but sometimes symptoms may not appear until years after the event. These symptoms cause significant problems in social or work situations and in relationships. They can also interfere with your ability to go about your normal daily tasks.
PTSD symptoms are generally grouped into four types: intrusive memories, avoidance, negative changes in thinking and mood, and changes in physical and emotional reactions. Symptoms can vary over time or vary from person to person.
Intrusive memories
Symptoms of intrusive memories may include:
- Recurrent, unwanted distressing memories of the traumatic event
- Reliving the traumatic event as if it were happening again (flashbacks)
- Upsetting dreams or nightmares about the traumatic event
- Severe emotional distress or physical reactions to something that reminds you of the traumatic event
Symptoms of avoidance may include:
- Trying to avoid thinking or talking about the traumatic event
- Avoiding places, activities or people that remind you of the traumatic event
Negative changes in thinking and mood
Symptoms of negative changes in thinking and mood may include:
- Negative thoughts about yourself, other people or the world
- Hopelessness about the future
- Memory problems, including not remembering important aspects of the traumatic event
- Difficulty maintaining close relationships
- Feeling detached from family and friends
- Lack of interest in activities you once enjoyed
- Difficulty experiencing positive emotions
- Feeling emotionally numb
Changes in physical and emotional reactions
Symptoms of changes in physical and emotional reactions (also called arousal symptoms) may include:
- Being easily startled or frightened
- Always being on guard for danger
- Self-destructive behavior, such as drinking too much or driving too fast
- Trouble sleeping
- Trouble concentrating
- Irritability, angry outbursts or aggressive behavior
- Overwhelming guilt or shame
For children 6 years old and younger, signs and symptoms may also include:
- Re-enacting the traumatic event or aspects of the traumatic event through play
- Frightening dreams that may or may not include aspects of the traumatic event
Intensity of symptoms
PTSD symptoms can vary in intensity over time. You may have more PTSD symptoms when you're stressed in general, or when you come across reminders of what you went through. For example, you may hear a car backfire and relive combat experiences. Or you may see a report on the news about a sexual assault and feel overcome by memories of your own assault.
When to see a doctor
If you have disturbing thoughts and feelings about a traumatic event for more than a month, if they're severe, or if you feel you're having trouble getting your life back under control, talk to your doctor or a mental health professional. Getting treatment as soon as possible can help prevent PTSD symptoms from getting worse.
If you have suicidal thoughts
If you or someone you know has suicidal thoughts, get help right away through one or more of these resources:
- Reach out to a close friend or loved one.
- Contact a minister, a spiritual leader or someone in your faith community.
- Contact a suicide hotline. In the U.S., call or text 988 to reach the 988 Suicide & Crisis Lifeline , available 24 hours a day, seven days a week. Or use the Lifeline Chat . Services are free and confidential.
- Make an appointment with your doctor or a mental health professional.
When to get emergency help
If you think you may hurt yourself or attempt suicide, call 911 or your local emergency number immediately.
If you know someone who's in danger of attempting suicide or has made a suicide attempt, make sure someone stays with that person to keep him or her safe . Call 911 or your local emergency number immediately. Or, if you can do so safely, take the person to the nearest hospital emergency room.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
You can develop post-traumatic stress disorder when you go through, see or learn about an event involving actual or threatened death, serious injury or sexual violation.
Doctors aren't sure why some people get PTSD. As with most mental health problems, PTSD is probably caused by a complex mix of:
- Stressful experiences, including the amount and severity of trauma you've gone through in your life
- Inherited mental health risks, such as a family history of anxiety and depression
- Inherited features of your personality — often called your temperament
- The way your brain regulates the chemicals and hormones your body releases in response to stress
Risk factors
People of all ages can have post-traumatic stress disorder. However, some factors may make you more likely to develop PTSD after a traumatic event, such as:
- Experiencing intense or long-lasting trauma
- Having experienced other trauma earlier in life, such as childhood abuse
- Having a job that increases your risk of being exposed to traumatic events, such as military personnel and first responders
- Having other mental health problems, such as anxiety or depression
- Having problems with substance misuse, such as excess drinking or drug use
- Lacking a good support system of family and friends
- Having blood relatives with mental health problems, including anxiety or depression
Kinds of traumatic events
The most common events leading to the development of PTSD include:
- Combat exposure
- Childhood physical abuse
- Sexual violence
- Physical assault
- Being threatened with a weapon
- An accident
Many other traumatic events also can lead to PTSD, such as fire, natural disaster, mugging, robbery, plane crash, torture, kidnapping, life-threatening medical diagnosis, terrorist attack, and other extreme or life-threatening events.
Complications
Post-traumatic stress disorder can disrupt your whole life — your job, your relationships, your health and your enjoyment of everyday activities.
Having PTSD may also increase your risk of other mental health problems, such as:
- Depression and anxiety
- Issues with drugs or alcohol use
- Eating disorders
- Suicidal thoughts and actions
After surviving a traumatic event, many people have PTSD-like symptoms at first, such as being unable to stop thinking about what's happened. Fear, anxiety, anger, depression, guilt — all are common reactions to trauma. However, the majority of people exposed to trauma do not develop long-term post-traumatic stress disorder.
Getting timely help and support may prevent normal stress reactions from getting worse and developing into PTSD. This may mean turning to family and friends who will listen and offer comfort. It may mean seeking out a mental health professional for a brief course of therapy. Some people may also find it helpful to turn to their faith community.
Support from others also may help prevent you from turning to unhealthy coping methods, such as misuse of alcohol or drugs.
- Posttraumatic stress disorder. In: Diagnostic and Statistical Manual of Mental Disorders DSM-5. 5th ed. Arlington, Va.: American Psychiatric Association; 2013. http://www.psychiatryonline.org. Accessed Dec.13, 2016.
- Clinician's guide to medications for PTSD. National Center for PTSD. http://www.ptsd.va.gov/professional/treatment/overview/clinicians-guide-to-medications-for-ptsd.asp. Accessed Dec. 13, 2016.
- Understanding PTSD and PTSD treatment. National Center for PTSD. http://www.ptsd.va.gov/public/PTSD-overview/basics/index.asp. Accessed Dec. 13, 2016.
- Treatment of PTSD. National Center for PTSD. http://www.ptsd.va.gov/public/treatment/therapy-med/treatment-ptsd.asp. Accessed Dec. 13, 2016.
- Coping with traumatic stress reactions. National Center for PTSD. http://www.ptsd.va.gov/public/treatment/cope/coping-traumatic-stress.asp. Accessed Dec. 13, 2016.
- Helping a family member who has PTSD. National Center for PTSD. http://www.ptsd.va.gov/public/family/helping-family-member.asp. Accessed Dec. 13, 2016.
- Post-traumatic stress disorder. National Institute of Mental Health. https://www.nimh.nih.gov/health/topics/post-traumatic-stress-disorder-ptsd/index.shtml. Accessed Dec. 13, 2016.
- Posttraumatic stress disorder. National Alliance on Mental Illness. https://www.nami.org/Learn-More/Mental-Health-Conditions/Posttraumatic-Stress-Disorder/Support. Accessed Dec. 13, 2016.
- Rothbaum BO. Psychotherapy for posttraumatic stress disorder in adults. http//www.uptodate.com/home. Accessed Dec. 13, 2016.
- What is posttraumatic stress disorder? American Psychiatric Association. https://www.psychiatry.org/patients-families/ptsd/what-is-ptsd. Accessed Dec. 13, 2016.
- Lifestyle changes recommended for PTSD patients. National Center for PTSD. http://www.ptsd.va.gov/public/treatment/cope/coping-ptsd-lifestyle-changes.asp. Accessed Dec. 13, 2016.
- Krieger CA (expert opinion). Mayo Clinic, Rochester, Minn. Jan. 10, 2017.
- Sawchuk CN (expert opinion). Mayo Clinic, Rochester, Minn. Jan. 13, 2017.
- Raskind MA, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. The New England Journal of Medicine. 2018;378:507.
- Hall-Flavin DK (expert opinion). Mayo Clinic, Rochester, Minn. June 27, 2018.
- Post-traumatic stress: How can you help your loved one?
Associated Procedures
- Acupuncture
- Cognitive behavioral therapy
- Psychotherapy
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Help transform healthcare
Your donation can make a difference in the future of healthcare. Give now to support Mayo Clinic's research.

ZACHARY SARTOR, MD, LANCE KELLEY, PhD, AND RYAN LASCHOBER, MD
Am Fam Physician. 2023;107(3):273-281
Patient information: See related handout on posttraumatic stress disorder , written by the authors of this article.
Author disclosure: No relevant financial relationships.
Posttraumatic stress disorder (PTSD) is common, with a lifetime prevalence of approximately 6%. PTSD may develop at least one month after a traumatic event involving the threat of death or harm to physical integrity, although earlier symptoms may represent an acute stress disorder. Symptoms typically involve trauma-related intrusive thoughts, avoidant behaviors, negative alterations of cognition or mood, and changes in arousal and reactivity. Assessing for past trauma in patients with anxiety or other psychiatric illnesses may aid in diagnosing and treating PTSD. The Diagnostic and Statistical Manual of Mental Disorders , 5th ed., text revision provides diagnostic criteria, and the PTSD Checklist for DSM-5 uses these diagnostic criteria to help physicians diagnose PTSD and determine severity. First-line treatment of PTSD involves psychotherapy, such as trauma-focused cognitive behavior therapy. Pharmacotherapy is useful for patients who have residual symptoms after psychotherapy or are unable or unwilling to access psychotherapy. Selective serotonin reuptake inhibitors (i.e., fluoxetine, paroxetine, and sertraline) and the serotonin-norepinephrine reuptake inhibitor venlafaxine effectively treat primary PTSD symptoms. The addition of other pharmacotherapy, such as atypical antipsychotics or topiramate, may be helpful for residual symptoms. Patients with PTSD often have sleep disturbance related to hyperarousal or nightmares. Prazosin is effective for the treatment of PTSD-related sleep disturbance. Clinicians should consider testing patients with PTSD for obstructive sleep apnea because many patients with PTSD-related sleep disturbance have this condition. Psychiatric comorbidities, particularly mood disorders and substance use, are common in PTSD and are best treated concurrently.
- Immediate, unlimited access to all AFP content
- More than 130 CME credits/year
- AAFP app access
- Print delivery available
Issue Access
- Immediate, unlimited access to this issue's content
- CME credits
Article Only
- Immediate, unlimited access to just this article
Gradus JL. Epidemiology of PTSD. National Center for PTSD; U.S. Department of Veterans Affairs. October 6, 2022. Accessed December 16, 2022. https://www.ptsd.va.gov/professional/treat/essentials/epidemiology.asp
National Institute of Mental Health. Post-Traumatic Stress Disorder. Accessed February 12, 2022. https://www.nimh.nih.gov/health/topics/post-traumatic-stress-disorder-ptsd
Spoont MR, Williams JW, Kehle-Forbes S, et al. Does this patient have posttraumatic stress disorder?: Rational clinical examination systematic review [published correction appears in JAMA . 2016; 315(1): 90]. JAMA. 2015;314(5):501-510.
Harvard Medical School. National Comorbidity Survey (NCS) . 2017. Accessed February 12, 2022. https://www.hcp.med.harvard.edu/ncs/index.php
Diagnostic and Statistical Manual of Mental Disorders . 5th ed., text revision. American Psychiatric Association; 2022.
Ryder AL, Azcarate PM, Cohen BE. PTSD and physical health. Curr Psychiatry Rep. 2018;20(12):116.
Bohnert KM, Sripada RK, Mach J, et al. Same-day integrated mental health care and PTSD diagnosis and treatment among VHA primary care patients with positive PTSD screens. Psychiatr Serv. 2016;67(1):94-100.
Kilpatrick DG, Resnick HS, Milanak ME, et al. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547.
Yehuda R, Hoge CW, McFarlane AC, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057.
Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
National Center for PTSD. How common is PTSD in adults? U.S. Department of Veterans Affairs. August 29, 2022. Accessed July 18, 2022. https://www.ptsd.va.gov/understand/common/common_adults.asp
Livingston NA, Berke D, Scholl J, et al. Addressing diversity in PTSD treatment: clinical considerations and guidance for the treatment of PTSD in LGBTQ populations. Curr Treat Options Psychiatry. 2020;7(2):53-69.
Yedlinsky NT, Neff LA, Jordan KM. Care of the military veteran: selected health issues. Am Fam Physician. 2019;100(9):544-551.
Rose S, Bisson J, Churchill R, et al. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2002(2):CD000560.
Roberts NP, Kitchiner NJ, Kenardy J, et al. Multiple session early psychological interventions for the prevention of post-traumatic stress disorder. Cochrane Database Syst Rev. 2019(8):CD006869.
Bertolini F, Robertson L, Bisson JI, et al. Early pharmacological interventions for universal prevention of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2022(2):CD013443.
Bryant RA. Acute stress disorder as a predictor of posttraumatic stress disorder: a systematic review. J Clin Psychiatry. 2011;72(2):233-239.
Kliem S, Kröger C. Prevention of chronic PTSD with early cognitive behavioral therapy. A meta-analysis using mixed-effects modeling. Behav Res Ther. 2013;51(11):753-761.
Jakob JMD, Lamp K, Rauch SAM, et al. The impact of trauma type or number of traumatic events on PTSD diagnosis and symptom severity in treatment seeking veterans. J Nerv Ment Dis. 2017;205(2):83-86.
National Center for PTSD. Clinician-administered PTSD scale for DSM-5 (CAPS-5). U.S. Department of Veterans Affairs. October 21, 2022. Accessed July 6, 2022. https://www.ptsd.va.gov/professional/assessment/adult-int/caps.asp
Weathers FW, Litz BT, Keane TM, et al. PTSD checklist for DSM-5 (PCL-5). April 11, 2018. Accessed February 12, 2022. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.PDF
LeardMann CA, McMaster HS, Warner S, et al. Comparison of posttraumatic stress disorder checklist instruments from Diagnostic and Statistical Manual of Mental Disorders , Fourth Edition vs Fifth Edition in a large cohort of US military service members and veterans. JAMA Netw Open. 2021;4(4):e218072.
Sareen J, Cox BJ, Stein MB, et al. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med. 2007;69(3):242-248.
Warner CH, Warner CM, Appenzeller GN, et al. Identifying and managing posttraumatic stress disorder [published correction appears in Am Fam Physician . 2014; 89(6): 424]. Am Fam Physician. 2013;88(12):827-834.
Katzman MA, Bleau P, Blier P, et al.; Canadian Anxiety Guidelines Initiative Group on behalf of the Anxiety Disorders Association of Canada/Association Canadienne des troubles anxieux and McGill University. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(suppl 1):S1.
Guideline Development Panel for the Treatment of PTSD in Adults. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. American Psychological Association; 2017. Accessed February 12, 2022. https://www.apa.org/ptsd-guideline/ptsd.pdf
The Management of Posttraumatic Stress Disorder Work Group. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. U.S. Department of Veterans Affairs/Department of Defense; 2017. Accessed February 12, 2022. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
National Institute for Health and Care Excellence. Post-traumatic stress disorder. NICE guideline [NG116]. December 5, 2018. Accessed February 12, 2022. https://www.nice.org.uk/guidance/ng116
Bisson JI, Roberts NP, Andrew M, et al. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev. 2013(12):CD003388.
Lee DJ, Schnitzlein CW, Wolf JP, et al. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta-analyses to determine first-line treatments. Depress Anxiety. 2016;33(9):792-806.
Merz J, Schwarzer G, Gerger H. Comparative efficacy and acceptability of pharmacological, psychotherapeutic, and combination treatments in adults with posttraumatic stress disorder: a network meta-analysis. JAMA Psychiatry. 2019;76(9):904-913.
Coventry PA, Meader N, Melton H, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262.
Forman-Hoffman V, Middleton JC, Feltner C, et al. Psychological and pharmacological treatments for adults with posttraumatic stress disorder: a systematic review update. Agency for Healthcare Research and Quality; 2018. Accessed February 12, 2022. https://effectivehealthcare.ahrq.gov/products/ptsd-adult-treatment-update/research-2018
National Center for PTSD. PTSD Treatment Basics. U.S. Department of Veterans Affairs. August 10, 2022. Accessed July 5, 2022. www.ptsd. va.gov/understand_tx/tx_basics.asp
Simon N, Robertson L, Lewis C, et al. Internet-based cognitive and behavioural therapies for post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev. 2021(5):CD011710.
Stewart CL, Wrobel TA. Evaluation of the efficacy of pharmacotherapy and psychotherapy in treatment of combat-related post-traumatic stress disorder: a meta-analytic review of outcome studies. Mil Med. 2009;174(5):460-469.
Williams T, Phillips NJ, Stein DJ, et al. Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2022(3):CD002795.
Rothbaum BO, Killeen TK, Davidson JRT, et al. Placebo-controlled trial of risperidone augmentation for selective serotonin reuptake inhibitor-resistant civilian posttraumatic stress disorder. J Clin Psychiatry. 2008;69(4):520-525.
Krystal JH, Rosenheck RA, Cramer JA, et al.; Veterans Affairs Cooperative Study No. 504 Group. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: a randomized trial. JAMA. 2011;306(5):493-502.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis [published correction appears in Lancet . 2019; 394(10202): 918]. Lancet. 2019;394(10202):939-951.
Robert S, Hamner MB, Durkalski VL, et al. An open-label assessment of aripiprazole in the treatment of PTSD. Psychopharmacol Bull. 2009;42(1):69-80.
Richardson JD, Fikretoglu D, Liu A, et al. Aripiprazole augmentation in the treatment of military-related PTSD with major depression: a retrospective chart review. BMC Psychiatry. 2011;11:86.
Batki SL, Pennington DL, Lasher B, et al. Topiramate treatment of alcohol use disorder in veterans with posttraumatic stress disorder: a randomized controlled pilot trial. Alcohol Clin Exp Res. 2014;38(8):2169-2177.
Qaseem A, Kansagara D, Forciea MA. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
Miller KE, Brownlow JA, Gehrman PR. Sleep in PTSD: treatment approaches and outcomes. Curr Opin Psychol. 2020;34:12-17.
Carlson GC, Kelly MR, Mitchell M, et al. Benefits of cognitive behavioral therapy for insomnia for women veterans with and without probable post-traumatic stress disorder. Womens Health Issues. 2022;32(2):194-202.
El-Solh AA, O'Brien N, Akinnusi M, et al. Predictors of cognitive behavioral therapy outcomes for insomnia in veterans with post-traumatic stress disorder. Sleep Breath. 2019;23(2):635-643.
Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Zhang Y, Ren R, Sanford LD, et al. The effects of prazosin on sleep disturbances in post-traumatic stress disorder: a systematic review and meta-analysis. Sleep Med. 2020;67:225-231.
Zhang Y, Weed JG, Ren R, et al. Prevalence of obstructive sleep apnea in patients with posttraumatic stress disorder and its impact on adherence to continuous positive airway pressure therapy: a meta-analysis. Sleep Med. 2017;36:125-132.
Zhang Y, Ren R, Yang L, et al. The effect of treating obstructive sleep apnea with continuous positive airway pressure on posttraumatic stress disorder: a systematic review and meta-analysis with hypothetical model. Neurosci Biobehav Rev. 2019;102:172-183.
El-Solh AA, Homish GG, Ditursi G, et al. A randomized crossover trial evaluating continuous positive airway pressure versus mandibular advancement device on health outcomes in veterans with posttraumatic stress disorder. J Clin Sleep Med. 2017;13(11):1327-1335.
Rytwinski NK, Scur MD, Feeny NC, et al. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J Trauma Stress. 2013;26(3):299-309.
Pietrzak RH, Goldstein RB, Southwick SM, et al. Prevalence and axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25(3):456-465.
Simpson TL, Rise P, Browne KC, et al. Clinical presentations, social functioning, and treatment receipt among individuals with comorbid lifetime PTSD and alcohol use disorders versus drug use disorders: findings from NESARC-III. Addiction. 2019;114(6):983-993.
Leeies M, Pagura J, Sareen J, et al. The use of alcohol and drugs to self-medicate symptoms of posttraumatic stress disorder. Depress Anxiety. 2010;27(8):731-736.
National Center for PTSD. Treatment of co-occurring PTSD and substance use disorder in VA. U.S. Department of Veterans Affairs. October 6, 2022. Accessed July 7, 2022. https://www.ptsd.va.gov/professional/treat/cooccurring/tx_sud_va.asp
Roberts NP, Roberts PA, Jones N, et al. Psychological therapies for post-traumatic stress disorder and comorbid substance use disorder. Cochrane Database Syst Rev. 2016(4):CD010204.
Simpson TL, Kaysen DL, Fleming CB, et al. Cognitive processing therapy for relapse prevention for comorbid posttraumatic stress disorder and alcohol use disorder: a randomized clinical trial. Plos One. 2022;17(11):e0276111.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2023 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Posttraumatic stress disorder.
Sukhmanjeet Kaur Mann ; Raman Marwaha ; Tyler J. Torrico .
Affiliations
Last Update: February 25, 2024 .
- Continuing Education Activity
Posttraumatic stress disorder (PTSD) is a prevalent and complex psychiatric condition that arises in response to exposure to traumatic events, significantly impacting an individual's mental well-being. Characterized by a diverse array of symptoms, PTSD can affect cognition, mood, somatic experiences, and behavior, leading to chronic impairments and an elevated risk of comorbid psychiatric illnesses, including an increased susceptibility to suicide. This activity describes the evaluation and management of PTSD and highlights the role of the interprofessional team in improving care for affected patients.
Clinicians participating in this activity can expect to gain comprehensive insights into the complexity of managing PTSD, acknowledging the individualized nature of trauma cases and the variability in symptom manifestation. Participants can anticipate learning about both psychological interventions and pharmacotherapy for prevention and treatment. Clinicians are provided a holistic approach to addressing the multifaceted challenges posed by this challenging psychiatric disorder.
- Identify the DSM-5-TR diagnostic criteria for PTSD.
- Differentiate between various presentations of PTSD by recognizing diverse symptomatology, considering cultural nuances, and understanding the impact of individualized trauma experiences.
- Implement evidence-based therapeutic interventions for PTSD, including cognitive-behavioral therapies, pharmacotherapy, and emerging treatments, tailoring approaches to individual patient needs.
- Collaborate with an interdisciplinary team to improve care coordination, addressing the multifaceted challenges posed by PTSD.
- Introduction
Posttraumatic stress disorder (PTSD) is a common psychiatric disorder that can result after an individual experiences a traumatic event. PTSD has a broad clinical presentation but is characterized by symptoms impairing cognition, mood, somatic experience, and behavior. PTSD can cause chronic impairments, lead to comorbid psychiatric illness, and lead to an increased risk of suicide. [1]
PTSD was first included in the Diagnostic and Statistical Manual of Mental Disorders (DSM) 3rd edition , published in 1980. [2] The inclusion of PTSD in the DSM reflects the acknowledgment of the significant impact that exposure to traumatic events can have on an individual's mental health. The DSM criteria for PTSD involve experiencing a traumatic event, the presence of specific symptoms such as intrusive memories or nightmares, avoidance behaviors, negative changes in mood and cognition, and heightened arousal. The inclusion of PTSD in the DSM has contributed to better understanding, diagnosis, and treatment of individuals who have experienced trauma. [1] The management of PTSD is complex, as each case of trauma is individualized, and specific symptoms of PTSD vary from case to case. Prevention and treatment methods involve psychological interventions as well as pharmacotherapy. [3] [4] [5]
Individuals who experience trauma may or may not develop long-term mental health sequela as a result of the trauma. However, the DSM-5-TR defines trauma as an essential characteristic of those who develop PTSD. Trauma (in the context of PTSD) is defined as exposure to actual or threatened death, serious injury, or sexual violence. This includes directly experiencing the traumatic event, witnessing a person experiencing trauma, or learning that the traumatic event occurred to a close family member or friend. [6]
There are various psychological theories proposed to explain trauma's capacity to cause PTSD. The shattered assumptions theory was proposed by Janoff-Bulman in 1992. [7] This theory suggests that traumatic events can change how individuals perceive themselves and the world as compared to their views before the traumatic experience. This theory has preliminary assumptions, including: "the world is benevolent," "the world is meaningful," and "the self is worthy." After trauma, the foundation for these inherited assumptions is weakened or "shattered." [7]
Psychodynamic psychology emphasizes the systematic study of how life experiences may relate to the current psychological forces on the mind, which impact behavior and emotions. [8] In 1890, Jean-Martin Charcot argued that psychological trauma was the origin of all mental illness. [9] Over time, this has been refuted, but it is acknowledged that trauma (and particularly early life trauma) can have a profound impact on the development of mental illness. A psychodynamic psychological view of posttraumatic stress relates particularly to unconscious decisions of trust. Individuals who experience trauma can have difficulty trusting that the world can be a safe place or trusting that individuals will not emotionally or physically harm them. [10]
Behavioral scientists have also contributed to understanding trauma's impact on cognitive processes. A conditioned response of learned fear can occur after exposure to a significant stimulus, which is usually the case in the context of PTSD. Individuals exposed to repeated traumas (such as those experiencing domestic or parental abuse) can develop a conditioned response to trauma. [11]
The presence or absence of support after trauma can both increase or decrease the risk of PTSD. Individuals who have a well-established support system are less likely to develop PTSD after a traumatic event. Likewise, individuals who feel isolated after trauma or have a poor social support system are more likely to develop an acute stress disorder and/or PTSD. [12] The risk of PTSD after a traumatic event is further increased by lower educational level, lower socioeconomic status, childhood adversity, gender, race, physical injury (including traumatic brain injury), and initial severity of the reaction to the trauma. [13] [14] [15] [16] [17]
- Epidemiology
The lifetime prevalence of PTSD ranges from 6.1% to 9.2% from national samples of the general adult population of the United States and Canada. [18] [13] [19] [20] The 1-year prevalence rates range from 3.5% to 4.7%. [20] [21] In the Western Hemisphere, certain populations have been found to have a higher prevalence of PTSD, including indigenous peoples and refugees. [22] [23] [13] Lower prevalence rates of PTSD have been found outside of the Western Hemisphere, but the reason for lower PTSD rates in the Eastern Hemisphere is not well understood. [24]
Intentional trauma has been found to have a greater association with PTSD than accidental trauma or nonviolent trauma. [25] [26] Repeated trauma and increasing duration of trauma exposure are also associated with a higher risk of PTSD. [27] Males and females both commonly develop PTSD after trauma, but females are known to be more predisposed to PTSD, with slight variations depending on the type of traumatic experience. [28]
- Pathophysiology
The initial response to trauma is associated with the pathophysiology of PTSD. The response is characterized by a surge of adrenaline from sympathetic nervous system stimulation. Physiologically, this can lead to tachycardia, rising blood pressure, and further neuroendocrine responses such as the release of cortisol and other catecholamines. [29] When the trauma stimulus is prolonged or repeated, a conditioned behavioral response leading to acute stress disorder or PTSD can occur.
Neuroanatomically, the amygdala has significant responsibility for threat detection and fear response. Magnetic resonance imaging (MRI) studies of individuals with PTSD have revealed nonspecific findings, including reduced total brain volume, although the results are not consistent. [30] [31] The amygdala is part of the ancient brain evolutionarily, meaning that its activation is primary and typically toned down by the frontal cortex as cognition and learned behaviors develop. [32] In patients with PTSD, the toning down capacity of the frontal lobe is dysregulated compared to those without PTSD. This observation may partially explain the imaging findings of reduced brain volume in those individuals with chronic PTSD. Neurotransmitter levels have been investigated in those with PTSD, including serotonin, dopamine, epinephrine, norepinephrine, glutamate, and gamma-aminobutyric acid (GABA). [33] Neurotransmitter levels in patients with PTSD have had inconsistent findings but still form the basis of an approach for treatment with psychotropic medications. [34]
- History and Physical
The presentation of PTSD is variable in both the history of the illness and the clinical symptomatology. Trauma is broad, and risks for certain types of trauma vary depending on patient characteristics such as age, gender, geographic location, family and marital status, and presence of a physical disability. [35] Types of trauma include sexual assault, mass political conflict and displacement (refugee), military or combat exposure, physical injury, and medical illness. [17] Due to the broad range of possible traumas, it is essential to understand individual patient backgrounds and social history. Additionally, adult patients with PTSD commonly suffer from symptoms as a result of childhood trauma, which can be far in the distant past compared to the time of clinical evaluation. [36] Duration of symptoms since the traumatic event is significant to note as this distinguishes PTSD from other psychiatric disorders (such as acute stress disorder). [37]
Dissociative symptoms may be present in patients with PTSD, and when these symptoms are present, they must be distinguished from a prior dissociative disorder. Dissociative symptoms include the following:
- Depersonalization: Feeling disconnected from one's body and feeling "lost" or "floating above my body."
- Derealization: Feeling as if the surrounding world is not real, such as watching the world from a dreamlike state. [38]
Discussing trauma with patients who are being evaluated for PTSD requires an approach with sensitivity. [39] In the context of sexual assault trauma, the gender of the provider and patient should be taken into consideration, as many patients who are survivors of sexual assault may have difficulty being in an interview room alone with the gender of their perpetrator. Some patients can talk about past trauma with ease, while others are not able to discuss details without experiencing acute symptoms. When engaging in a discussion of trauma details, it is important to respect patient boundaries on the topic and ask how deeply or superficially the patient prefers to discuss the topic. These are foundational concepts of trauma-informed interventions. [39] Notably, the specific details of the trauma are usually not necessary for obtaining a PTSD diagnosis. Specific details of trauma are only necessary for certain types of psychotherapeutic treatments, which the patient should consent to before initiating. General questioning about symptoms related to trauma is usually an optimal approach for a first diagnostic interview where developing therapeutic rapport is essential. [40] General questions can include the following:
- Do you think about the traumatic event more than you would like to?
- Do you have nightmares or flashbacks related to the trauma?
- Do you avoid people or triggers associated with the trauma?
- Are you struggling with feelings of persistent sadness?
The mental status examination (MSE), conducted during psychiatric evaluations, is crucial in assessing individuals with PTSD. [41] However, it is necessary to note that the specific elements and findings of the examination can vary depending on each case of PTSD. Components of the MSE should include the following:
- Appearance: Scars, wounds, and other deformities may be present due to prior traumatic experiences.
- Attitude and Behavior: PTSD can commonly lead to hypervigilant behavior. Eye contact should observed.
- Affect: Patients with PTSD may present fearful, anxious, apathetic, or depressed. Affect may change depending on the conversation, and the range of affect should be observed. PTSD may present with constricted affect consistent with feeling numb.
- Thought content: Thought content should be evaluated to assess suicide ideations and self-harm behaviors.
- Thought process: For patients with persistent and exaggerated negative beliefs after trauma, the thought process may deviate from linear.
- Insight: Patients with PTSD commonly have a fair understanding of their illness, although specific populations of patients may minimize their symptoms. Patients with PTSD may have difficulty understanding how their PTSD symptoms relate to potential other psychiatric comorbidity (such as major depression, substance use disorders, and borderline personality disorder).
- Judgment: Judgment can be assessed in individuals with PTSD based on their clinical presentation as well as their ability to make rational decisions related to their treatment plan options. [42]
Physical examination findings in patients with PTSD are typically nonspecific and often do not reveal overt physiological abnormalities. While PTSD primarily manifests as a psychiatric condition, some individuals may exhibit physical symptoms related to heightened arousal or chronic stress. The exam findings may include increased heart rate, elevated blood pressure, muscle tension, and disrupted sleep patterns. While discussing the trauma specifically or during flashbacks, patients with PTSD may exhibit the above findings.
When patients are prescribed medications for PTSD that impact blood pressure (ie, clonidine, prazosin, and venlafaxine), it is essential to monitor blood pressure when considering medication adjustments. [43]
The psychiatric evaluation is the most important component of diagnosing PTSD. However, healthcare professionals can use validated rating scales to screen and diagnose PTSD, which is particularly helpful in settings where psychiatric specialists are not available. Self-report scales used in screening for PTSD include the PTSD Checklist for DSM-5 (PCL-5) and Trauma Symptom Checklist-40 (TSC-40). [44] [45] The Clinician-Administers PTSD scale is also available as a 30-item structured interview. [46]
To obtain a formal diagnosis of PTSD, individuals must meet the diagnostic criteria specific in the DSM-5-TR. The diagnosis involves a thorough evaluation that considers multiple sources of information, including personal history, collateral information, and an MSE. This comprehensive assessment allows clinicians to assess the individual's symptoms, functioning, and overall presentation concerning the established diagnostic criteria.
The diagnostic criteria and specifications listed below apply to adults and children older than 6 years. A preschool subtype of PTSD for children 6 years and younger is included in the DSM-5.
Posttraumatic Stress Disorder DSM-5-TR Criteria
Criterion A: Stressor
Exposure to real or threatened death, injury, or sexual violence in 1 or more of the following ways:
- Direct exposure to the traumatic event
- Witnessing the trauma as it occurred to someone else
- Learning about a close family relative or close friend being exposed to actual or threatened trauma, accidental or violent death
- Indirect exposure to distressing details of the traumatic event (professionals repeatedly exposed to the details of child abuse, collecting human remains, or pieces of evidence). This does not include exposure through television, movies, electronic devices, or pictures.
Criterion B: Intrusion Symptoms
Presence of 1 or more of the following symptoms related to the traumatic event and began after the trauma occurred:
- Recurrent, involuntary, and intrusive thoughts associated with the traumatic event. In children older than 6 years, this may be expressed using repetitive play in which the aspects of the trauma are expressed.
- Distressing nightmares may be repetitive, and the dream's content is related to the traumatic event. Children may have frightening dreams where they may or may not recognize the content.
- Dissociative reactions, such as flashbacks, in which the individual may feel or act that the traumatic event is happening again. These reactions may occur as a continuum ranging from brief responses to complete loss of awareness of oneself or the surroundings. Children may reenact such events in the play.
- Intense or prolonged psychological distress on exposure to traumatic reminders
- Marked physiological reactivity such as increased heart rate and blood pressure on exposure to traumatic reminders
Criterion C: Avoidance
Persistent avoidance of the stimuli related to the traumatic event, as evidenced by 1 or both of the following:
- Avoidance or efforts to avoid distressing memories or thoughts associated with the traumatic event
- Avoidance or efforts to avoid external reminders such as people, places, activities, conversations, or situations that arouse distressing memories or thoughts related to the traumatic event
Criterion D: Negative Alterations in Mood
Negative alterations in mood and cognition that began or worsened after the traumatic event, as evidenced by 2 or more of the following:
- Inability to recall important aspects of the traumatic event. This can be due to dissociative amnesia, not due to head injury, drugs, or alcohol.
- Persistent and distorted negative beliefs or expectations about oneself or the world, such as "I am bad" or "The world is completely dangerous."
- Persistent distorted cognition leads the individual to blame self or others for causing the traumatic event
- Persistent negative emotional state, including fear, guilt, anger, or shame
- Markedly diminished interest in significant activities that used to be enjoyable
- Feeling alienated, estranged, or detached from others
- Persistent inability to experience a positive emotion such as happiness, satisfaction, or love
Criterion E: Alterations in Arousal and Reactivity
Trauma-related alterations in reactivity and arousal that began or worsened after the traumatic event, as evidenced by 2 or more of the following:
- Irritable or aggressive outbursts with little or no provocation
- Reckless or self-destructive behavior
- Hypervigilance
- Exaggerated startle response
- Problems in concentration
- Sleep disturbances (difficulty falling or staying asleep, restless sleep)
Criterion F: Duration
Persistence of symptoms in Criterion B, C, D, and E for more than 1 month
Criterion G: The disturbance causes significant functional impairment or distress in various areas of life, such as social or occupational.
Criterion H: The disturbance is not attributable to substance use, medication, or another medical illness. [47]
Two specifications
Two PTSD specifiers are noted in the DSM-5: delayed expression and dissociative symptoms. The complete diagnostic criteria for PTSD must be satisfied before either specifier can be assigned.
Delayed Expression: Full diagnostic criteria are not satisfied until at or after 6 months from the target trauma, although some symptom onset may occur sooner. In some instances, the affected individual does not meet the full criteria for PTSD until years after the trauma. Military members have higher rates of delayed expression PTSD.
Dissociative Symptoms: Higher levels of recurrent or persistent depersonalization (feeling outside one's body or mind) or derealization (experiencing that things are not real) symptoms exist. Individuals with the dissociative specification of PTSD tend to have higher levels of exposure to childhood and/or adult sexual assault. Generally, they are more symptomatic than those without the specification. These patients also exhibit higher rates of functional impairment, psychiatric comorbidity, and suicidality.
- Treatment / Management
The treatment of PTSD requires a patient-specific approach, with the patient's consent for any treatment. Many patients with PTSD are unwilling to pursue treatment, and some patients have symptoms resistant to treatment. It may be necessary to use a combination of medications and therapy in certain patients; however, patients should be offered a choice of treatment preference between the two modalities. Therapy-based approaches are generally preferred, but patients with severe symptoms or comorbid illness may not be able to engage in meaningful therapy treatments initially and can be started on a medication treatment plan with an intent to integrate therapy in the future when the patient is more clinically stable.
Psychotherapeutic Approaches
Trauma-focused psychotherapy is the preferred treatment for PTSD. This includes cognitive behavioral therapy, exposure-based therapy, and eye movement desensitization and reprocessing therapy (EMDR). [3] [48] [49] [50] Clinical studies of patients who receive trauma-focused psychotherapy have demonstrated greater improvement in symptoms compared to those who do not receive treatment. [3] When trauma-focused psychotherapy is compared against pharmacotherapy for PTSD, there may be slightly improved outcomes with therapy. [51] [48]
Cognitive behavioral therapy utilizes techniques to identify and correct distorted maladaptive beliefs, which can occur after a traumatic event. Specific techniques include education, relaxation exercises, the use of coping skills, and stress management. [52]
Exposure-based therapy is a technique most commonly used to treat anxiety disorders such as specific phobias. The method considers a conditioned fear response from learned behavior and involves a measured approach of reintroducing the stimulus to lead to fear extinction eventually. With regard to PTSD, this requires patient consent for treatment, is not an applicable option for certain cases, and requires an intense workload on the patient. [53]
EMDR was developed after recognizing that certain saccadic eye movements reduce the intensity of disturbing thoughts. These eye movements can be voluntarily adjusted while thinking about a distressing memory, reducing the anxiety associated with it. EMDR has been shown to desensitize traumatic memories and has improved the appraised validity of a positive self-belief in those with PTSD. [54] [55] The therapeutic neural mechanisms of EMDR remain unclear. [56]
Supportive psychotherapy can be helpful in individuals who are dealing with acute trauma and those who have acute stress disorder. [57]
Medication Approaches
Selective serotonin reuptake inhibitors (SSRI) such as sertraline and paroxetine are FDA-cleared for the treatment of PTSD. Other SSRIs and selective serotonin and norepinephrine reuptake inhibitors (SNRI) are reasonable off-label alternatives. SSRIs have been found to reduce PTSD symptoms greater than placebo, but there is no strong evidence to differentiate the effectiveness of specific SSRIs and SNRIs. [4] [5] [58]
For patients with prominent sleep disturbances or nightmares associated with PTSD, off-label medication treatment approaches are commonly used. Prazosin is commonly used as monotherapy or in combination with an on-label medication treatment such as an SSRI. Prazosin competitively inhibits postsynaptic alpha-adrenergic receptors, resulting in vasodilation of veins and arterioles, decreasing blood pressure. When used in PTSD-associated nightmares, the hypothesis for the mechanism of action is a toned-down sympathetic response, which can decrease the frequency or severity of nightmares. However, there are mixed results for prazosin efficacy for this specific use, and the findings are inconsistent. [59] [60] [61] Clonidine is occasionally used for similar purposes. In patients prescribed blood pressure medications for PTSD, monitoring blood pressure at clinical visits is important, as well as avoiding suddenly stopping the medication to avoid rebound hypertension.
Other medications are less commonly used for treating PTSD (off-label), but second-generation antipsychotics are occasionally used. This approach can be helpful in patients who have comorbid psychotic symptoms or treatment-resistant depression (in which antipsychotics are commonly used to augment SSRIs). Quetiapine has shown efficacy as a monotherapy for PTSD in military veterans. [62] Other antipsychotics used in other populations with PTSD have limited and mixed results. [63] [64] [65]
Novel Approaches
In 2020, the FDA cleared a class II medical device that uses the hardware of common smart-watches to monitor heart rate during sleep for individuals with PTSD with the goal of correlating physiologic response (biofeedback) to PTSD-related nightmares. [66]
- Differential Diagnosis
Potential differential diagnoses of PTSD are listed below.
Acute Stress Disorder
The symptoms of PTSD and acute stress disorder significantly overlap. The onset and duration of the symptoms help in making the final diagnosis. Acute stress disorder is diagnosed if the symptoms are present for less than 1 month. [37]
Dissociative Disorders
Primary dissociative disorders include dissociative identity disorder (DID), dissociative amnesia, and depersonalization/derealization disorder. DID entails the disruption of identity characterized by 2 or more distinct personality states. Dissociative amnesia describes an inability to recall important autobiographical information; notably, the information is usually of a traumatic or stressful nature, but for diagnosis of dissociative amnesia, there are no other symptoms of PTSD. Depersonalization/derealization disorder shares symptoms of dissociation with PTSD but is without the other symptomatology of PTSD. [67]
Major Depressive Disorder
Affective changes are common in PTSD, and major depressive disorder (MDD) can be a comorbid condition with PTSD. Diagnosis of MDD requires at least 1 major depressive episode, which is persistent decreased mood for at least 2 weeks. [68]
Adjustment Disorder
Adjustment disorder describes the development of emotional or behavioral symptoms in response to an identifiable stressor (not necessarily trauma), occurring within 3 months of the stressor's onset. The symptoms may not persist for more than an additional 6 months and are otherwise considered to be classified to a more fitting chronic psychiatric diagnosis. [69]
Other Psychiatric Disorders
PTSD is a disorder with a variable duration of symptoms. Over time, patients may no longer meet the diagnostic criteria for PTSD if their symptoms in this domain improve. However, they may still be suffering from other psychiatric disorders and receiving mental health care. Patients with a history of improved or resolved PTSD may over-attribute PTSD as a primary disorder despite no longer meeting the diagnostic criteria. Clinicians should be mindful to assess if PTSD is current, improved, resolved, or comorbid with other psychiatric disorders. [70]
PTSD outcomes vary broadly from case to case due to many factors. Those who engage in PTSD treatments tend to have improved outcomes compared to those who do not engage in treatment. [3] [4] [5] Chronic PTSD is common, with estimates that one-third of patients still have symptoms 1 year after diagnosis, and another third of patients still have symptoms 10 years after diagnosis. [26]
Positive psychology emphasizes psychological resilience after trauma and posttraumatic growth. [71] These concepts describe positive changes in self-perception, interpersonal relationships, and philosophy of life that can occur for individuals who recover from trauma and PTSD. These strengths can lead to increased self-awareness, self-confidence, open attitudes, and appreciation for life. [72] Posttraumatic growth is optimal but not a guaranteed outcome; in fact, it may even be an uncommon outcome. Research in positive psychology applications to trauma disorders remains limited and in need of further study. [73] [74]
- Complications
Although they can resolve, PTSD symptoms may also lead to the development of other psychiatric comorbidity. Trauma is a known risk factor for MDD, borderline personality disorder, anxiety disorders, substance use disorders, psychotic disorders, and more. [75] Patients with PTSD are at increased risk for suicide and should have regular screenings for suicidal ideation by clinicians. [1] Individuals with PTSD are more likely to experience occupational problems than those without PTSD and have higher rates of disability. [76] Additionally, those with a history of sexual trauma report higher rates of problems with intimate relationships. [77] [76]
- Deterrence and Patient Education
Deterrence and prevention strategies for PTSD focus on minimizing the impact of traumatic events and mitigating the development of persistent psychological distress. Primary prevention efforts involve promoting resilience, coping skills, and social support networks to enhance individuals' ability to cope with stressors effectively. Clinician awareness for patient populations who may need screening for PTSD is essential for the detection of illness. Military personnel and veterans should be systematically screened for PTSD. [78] Primary care providers should be mindful of patients presenting with new anxiety, fear, and insomnia, which can be a result of trauma. [79] Community-based education on trauma-informed practices and early intervention initiatives can contribute to creating environments that reduce the risk of trauma exposure and mitigate its effects.
Secondary prevention emphasizes timely and targeted interventions for individuals at higher risk, such as those with a history of trauma or in high-stress professions, to prevent the escalation of symptoms. Integrating trauma-focused mental health awareness into various sectors, including education, healthcare, and emergency services, is essential for fostering a culture of prevention and support. By addressing risk factors and promoting resilience on individual and societal levels, the aim is to reduce the incidence and severity of PTSD, ultimately contributing to better mental health outcomes.
- Enhancing Healthcare Team Outcomes
PTSD is a common but complex psychiatric condition that can result after an individual experiences a traumatic event. Patients with PTSD benefit from referral to psychiatric specialists when available, but screening tools are available for clinicians in all settings to use in patients with whom they suspect PTSD. Physicians, advanced care practitioners, nurses, pharmacists, physical and occupational therapists, social workers, and other healthcare professionals are essential in making a collective commitment to enhancing patient-centered care, outcomes, patient safety, and team performance related to PTSD.
Proficiency in trauma-informed care and evidence-based interventions for PTSD is crucial. Clinicians should be adept at conducting comprehensive assessments, differentiating PTSD presentations, and tailoring therapeutic approaches to individual needs. Treatment involves psychotherapeutic interventions, primarily cognitive-behavioral therapy and pharmacotherapy, focusing on SSRIs with some evidence for the use of other medication classes. Including the patient's perspective and determining the appropriate care goals with an individual with PTSD is essential when using a trauma-informed approach.
A strategic approach involves the development and implementation of interdisciplinary care plans that address the multifaceted nature of PTSD. This includes collaboration on prevention strategies, early intervention, and long-term management, considering both pharmacological and psychological treatments. Ethical considerations emphasize sensitivity to the unique experiences of individuals with PTSD, respecting confidentiality, and maintaining cultural competence. Professionals should prioritize autonomy and informed consent while delivering patient-centered care.
Interprofessional communication is pivotal for a holistic approach. Clear and empathetic communication ensures a shared understanding of patients' needs, fostering a collaborative environment that integrates perspectives from diverse healthcare disciplines. Care coordination involves aligning efforts across the healthcare team to provide seamless and continuous support for patients with PTSD. This includes facilitating referrals, sharing relevant information, and ensuring a patient's journey through the healthcare system is cohesive and patient-centric. Collaboration with social workers, therapists, and family to optimize the social factors in a patient's life can offer significant stability to individuals with PTSD.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Sukhmanjeet Kaur Mann declares no relevant financial relationships with ineligible companies.
Disclosure: Raman Marwaha declares no relevant financial relationships with ineligible companies.
Disclosure: Tyler Torrico declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Mann SK, Marwaha R, Torrico TJ. Posttraumatic Stress Disorder. [Updated 2024 Feb 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Posttraumatic Stress Disorder - StatPearls Posttraumatic Stress Disorder - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

COMMENTS
Philip, a 60-year-old who was in a traffic accident (PDF, 294KB) This case example from the European Journal of Psychotraumatology details an assisted self-study application of cognitive therapy for PTSD. Philip developed PTSD and comorbid major depression following a traffic accident. He was treated in six sessions of cognitive therapy with ...
Monson, C. M. & Shnaider, P. (2014). Treating PTSD with cognitive-behavioral therapies: Interventions that work. Washington, DC: American Psychological Association. Updated July 31, 2017. Date created: 2017. This case example explains how Jill's therapist used a cognitive intervention with a written worksheet as a starting point for engaging in ...
The first case example about Terry documents the treatment of PTSD using Prolonged Exposure. The second is an example of in-session imaginal exposure with a different client. Prolonged Exposure is strongly recommended by the APA Clinical Practice Guideline for the Treatment of PTSD. Download case study (PDF, 107KB).
To illustrate IPT for PTSD, we present data from a pilot case that was not included in the current NIMH study, but served as a valuable training case. This patient received open IPT treatment for PTSD because his Clinician-Administered PTSD Scale (CAPS, Blake et al., 1995 ) score of 45, while indicating moderate symptom severity ( Weathers et ...
In conclusion, this case study demonstrates the possibility of using self-study modules in the treatment of PTSD despite the memory and concentration problems observed in this patient group. Of course, the conclusions that can be drawn from a case study are very limited as it is unknown whether the treatment was causally responsible for Philip ...
A large meta-analysis in 2013 reviewed all available research on efficacy of treatments and interventions for PTSD, with a total of 112 non-duplicate studies included. This existing body of research was influential in the formation and updates to the VA and DoD clinical practice guidelines for assessment and treatment of PTSD in that same year.
As is the case with hippocampal size, sleep difficulties might be both a risk factor and a symptom of PTSD. Studies in military and civilian populations have reported an association between the ...
Posttraumatic stress disorder (PTSD) is a chronic, often debilitating mental health disorder that may develop after a traumatic life event. Fortunately, effective psychological treatments for PTSD exist. In 2017, the Veterans Health Administration and Department of Defense (VA/DoD) and the American Psychological Association (APA) each published ...
Study participants. This case-control pilot study involved 40 (20 controls; 20 PTSD) age and sex-matched participants recruited in the US between January and June 2019, by Discovery Life Sciences (DLS), California USA, and PrecisionMed, California, USA. Venous blood samples and a detailed clinical history were collected from each study participant.
The Journal of Child and Family Studies states that there have been more mass shootings within the last 18 years than in the entire 20th century combined, with 77% carried out by adolescents. This case study aims to evaluate the clinical presentation of post-traumatic stress disorder (PTSD) in an adolescent by highlighting the clinical course of a school shooting survivor.
Instead of feeling shame and guilt, he said, "I can carry the memory with pride.". Case presentation written by Drs. E.C. Hurley, Louise Maxfield, and Roger M. Solomon. This is a case example for the treatment of PTSD using Eye Movement Desensitization and Reprocessing (EMDR) therapy.
3. Results. Here, we present seven cases from an open-label pilot study of the reconsolidation intervention for veterans with PTSD. The patients were on the waiting list for trauma-focused treatment at ARQ Centrum '45, when offered the possibility of this intervention.
This case study follows a 7-year-old boy who presented with symptoms of posttraumatic stress disorder (PTSD) following exposure to domestic violence beginning at a very young age.
Abstract. Post-traumatic stress disorder (PTSD), previously known as battle fatigue syndrome or shell shock, is a severe mental disturbance condition that is normally triggered by the experience of some frightening/scary events or trauma where a person undergoes some serious physical or mental harm or threatened.
The second case is the victim of a terrible accident at work, causing extensive burns, life-threatening complications, and disfiguring scars. Previous studies indicate that PTSD may be identified in up to 30% of such patients, stressing the need for a dedicated staff psychiatrist in modern burn centers 21, 22).
Request an Appointment. 410-955-5000 Maryland. 855-695-4872 Outside of Maryland. +1-410-502-7683 International. Posttraumatic stress disorder is debilitating anxiety that can affect people who have been through or witnessed a traumatic event. Counseling and medication can help.
Acknowledgment. This casebook offers detailed guidance to help practitioners understand and implement the treatments recommended in APA's Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder in Adults. The authors describe the unique factors involved in PTSD treatment, and core competencies necessary for providers.
This case study depicts using CPT for treating uncomplicated PTSD in a 28-year-old woman, while integrating brief exposure exercises in the client's homework to reflect the utility of integrating other evidence-based exercises into manualized treatment to create nuanced, individualized treatment plans. Posttraumatic stress disorder (PTSD) is a prevalent and concerning mental health diagnosis ...
Overview. Post-traumatic stress disorder (PTSD) is a mental health condition that's triggered by a terrifying event — either experiencing it or witnessing it. Symptoms may include flashbacks, nightmares and severe anxiety, as well as uncontrollable thoughts about the event. Most people who go through traumatic events may have temporary ...
A VR-GET case study reported a 20% reduction in the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) - Military Version (PCL-M) score in association with 10 VR-GET sessions for a Navy corpsman . Another VR-GET case study documented that 20 VR-GET sessions conducted with a Seabee, who had ...
TABLE 1.1. Summary of Recommendations of the APA Guideline Development Panel for the Treatment of Posttraumatic Stress Disorder (PTSD) (Continued) There is insufficient evidence to recommend for or against clinicians offering the following medications (listed alphabetically) for treatment of adults with PTSD.
Posttraumatic stress disorder (PTSD) is common, with a lifetime prevalence of approximately 6%. PTSD may develop at least one month after a traumatic event involving the threat of death or harm to ...
PREVALENCE. Although many people are exposed to traumatic events at some point in their lives, most of them rebound to enjoy pre‐trauma levels of psychological functioning18.Epidemiological studies have reported lifetime PTSD prevalence rates of 13.0‐20.4% for women and 6.2‐8.2% for men19, 20.The World Mental Health Surveys have observed higher 12‐month prevalence rates in high ...
"Our goal is to unlock sleep as a new treatment window for PTSD," says Hein van Marle, one of the paper's senior authors and the study's principal investigator at Amsterdam University Medical Center.
Posttraumatic stress disorder (PTSD) is a common psychiatric disorder that can result after an individual experiences a traumatic event. PTSD has a broad clinical presentation but is characterized by symptoms impairing cognition, mood, somatic experience, and behavior. PTSD can cause chronic impairments, lead to comorbid psychiatric illness, and lead to an increased risk of suicide.[1]