Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

CDC provides national leadership for HIV prevention research, including the development and evaluation of HIV biomedical and behavioral interventions to prevent HIV transmission and reduce HIV disease progression in the United States and internationally. CDC’s research efforts also include identifying those scientifically proven, cost-effective, and scalable interventions and prevention strategies to be implemented as part of a high-impact prevention approach for maximal impact on the HIV epidemic.
The AIDS epidemic, although first recognized only 20 years ago, has had a profound impact in communities throughout the United States.
The Serostatus Approach to Fighting the HIV Epidemic: Prevention Strategies for Infected Individuals R. S. Janssen, D. R. Holtgrave, and K. M. De Cock led the writing of this commentary. R. O. Valdiserri, M. Shepherd, and H. D. Gayle contributed ideas and helped with writing and reviewing the manuscript.

CDC has provided funding to HIV partners to help implement programs that will help curb the increase of HIV infections. These programs facilitated with our partners and grantees are critical in the goal of eliminating HIV infection in the United States.

CDC has researched several HIV prevention interventions that have proven effective in helping to prevent HIV infection in certain populations and communities.

CDC has worked with key cities to create effective policies and programs to curb the tide of HIV infections in those cities. These cities have higher rates of HIV due to a number of factors therefore making them key locations for studies.

The Medical Monitoring Project (MMP) is a surveillance system designed to learn more about the experiences and needs of people who are living with HIV. It is supported by several government agencies and conducted by state and local health departments along with the Centers for Disease Control and Prevention.
- Assessment of 2010 CDC-funded Health Department HIV Testing Spending and Outcomes [PDF – 359 KB]
- HIV Testing Trends in the United States, 2000-2011 [PDF – 1 MB]
- HIV Testing at CDC-Funded Sites, United States, Puerto Rico, and the U.S. Virgin Islands, 2010 [PDF – 691 KB]
- HIV Prevention Funding Allocations at CDC-Funded State and Local Health Departments, 2010 [PDF – 792 KB]

Cost-effectiveness of HIV Prevention
- The cost-effectiveness of HIV prevention efforts has long been a criterion in setting program priorities. The basic principle is straightforward: choose those options that provide the greatest outcome for the least cost.
- The fact sheet Projecting Possible Future Courses of the HIV Epidemic in the United States compares the cost-effectiveness of three different prevention investment scenarios.
The HIV/AIDS Prevention Research Synthesis (PRS) Project identifies evidence-based HIV behavioral interventions (EBIs) listed in the Compendium of Evidence-Based HIV Behavioral Interventions to help HIV prevention planners and providers in the United States choose the interventions most appropriate for their communities.
- On January 1, 2012, CDC began a new 5-year HIV prevention funding cycle with health departments, awarding $339 million annually.
- The STD/HIV National Network of Prevention Training Centers provides training for health departments and CBOs on the HIV prevention interventions.
- HIV Nexus: Resources for Clinicians
- Ending the HIV Epidemic (EHE)
- Public Health Partners
- Let’s Stop HIV Together
- @StopHIVTogether
- Get Email Updates
- Send Feedback
- Fact sheets
Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Observatory
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Fact sheets /
HIV and AIDS
- HIV remains a major global public health issue, having claimed an estimated 42.3 million lives to date. Transmission is ongoing in all countries globally.
- There were an estimated 39.9 million people living with HIV at the end of 2023, 65% of whom are in the WHO African Region.
- In 2023, an estimated 630 000 people died from HIV-related causes and an estimated 1.3 million people acquired HIV.
- There is no cure for HIV infection. However, with access to effective HIV prevention, diagnosis, treatment and care, including for opportunistic infections, HIV infection has become a manageable chronic health condition, enabling people living with HIV to lead long and healthy lives.
- WHO, the Global Fund and UNAIDS all have global HIV strategies that are aligned with the SDG target 3.3 of ending the HIV epidemic by 2030.
- By 2025, 95% of all people living with HIV should have a diagnosis, 95% of whom should be taking lifesaving antiretroviral treatment, and 95% of people living with HIV on treatment should achieve a suppressed viral load for the benefit of the person’s health and for reducing onward HIV transmission. In 2023, these percentages were 86%, 89%, and 93% respectively.
- In 2023, of all people living with HIV, 86% knew their status, 77% were receiving antiretroviral therapy and 72% had suppressed viral loads.
Human immunodeficiency virus (HIV) is a virus that attacks the body’s immune system. Acquired immunodeficiency syndrome (AIDS) occurs at the most advanced stage of infection.
HIV targets the body’s white blood cells, weakening the immune system. This makes it easier to get sick with diseases like tuberculosis, infections and some cancers.
HIV is spread from the body fluids of an infected person, including blood, breast milk, semen and vaginal fluids. It is not spread by kisses, hugs or sharing food. It can also spread from a mother to her baby.
HIV can be prevented and treated with antiretroviral therapy (ART). Untreated HIV can progress to AIDS, often after many years.
WHO now defines Advanced HIV Disease (AHD) as CD4 cell count less than 200 cells/mm3 or WHO stage 3 or 4 in adults and adolescents. All children younger than 5 years of age living with HIV are considered to have advanced HIV disease.
Signs and symptoms
The symptoms of HIV vary depending on the stage of infection.
HIV spreads more easily in the first few months after a person is infected, but many are unaware of their status until the later stages. In the first few weeks after being infected people may not experience symptoms. Others may have an influenza-like illness including:
- sore throat.
The infection progressively weakens the immune system. This can cause other signs and symptoms:
- swollen lymph nodes
- weight loss
Without treatment, people living with HIV infection can also develop severe illnesses:
- tuberculosis (TB)
- cryptococcal meningitis
- severe bacterial infections
- cancers such as lymphomas and Kaposi's sarcoma.
HIV causes other infections to get worse, such as hepatitis C, hepatitis B and mpox.
Transmission
HIV can be transmitted via the exchange of body fluids from people living with HIV, including blood, breast milk, semen, and vaginal secretions. HIV can also be transmitted to a child during pregnancy and delivery. People cannot become infected with HIV through ordinary day-to-day contact such as kissing, hugging, shaking hands, or sharing personal objects, food or water.
People living with HIV who are taking ART and have an undetectable viral load will not transmit HIV to their sexual partners. Early access to ART and support to remain on treatment is therefore critical not only to improve the health of people living with HIV but also to prevent HIV transmission.
Risk factors
Behaviours and conditions that put people at greater risk of contracting HIV include:
- having anal or vaginal sex without a condom;
- having another sexually transmitted infection (STI) such as syphilis, herpes, chlamydia, gonorrhoea and bacterial vaginosis;
- harmful use of alcohol or drugs in the context of sexual behaviour;
- sharing contaminated needles, syringes and other injecting equipment, or drug solutions when injecting drugs;
- receiving unsafe injections, blood transfusions, or tissue transplantation; and
- medical procedures that involve unsterile cutting or piercing; or accidental needle stick injuries, including among health workers.
HIV can be diagnosed through rapid diagnostic tests that provide same-day results. This greatly facilitates early diagnosis and linkage with treatment and prevention. People can also use HIV self-tests to test themselves. However, no single test can provide a full HIV positive diagnosis; confirmatory testing is required, conducted by a qualified and trained health worker or community worker. HIV infection can be detected with great accuracy using WHO prequalified tests within a nationally approved testing strategy and algorithm.
Most widely used HIV diagnostic tests detect antibodies produced by a person as part of their immune response to fight HIV. In most cases, people develop antibodies to HIV within 28 days of infection. During this time, people are in the so-called “window period” when they have low levels of antibodies which cannot be detected by many rapid tests, but they may still transmit HIV to others. People who have had a recent high-risk exposure and test negative can have a further test after 28 days.
Following a positive diagnosis, people should be retested before they are enrolled in treatment and care to rule out any potential testing or reporting error. While testing for adolescents and adults has been made simple and efficient, this is not the case for babies born to HIV-positive mothers. For children less than 18 months of age, rapid antibody testing is not sufficient to identify HIV infection – virological testing must be provided as early as birth or at 6 weeks of age. New technologies are now available to perform this test at the point of care and enable same-day results, which will accelerate appropriate linkage with treatment and care.
HIV is a preventable disease. Reduce the risk of HIV infection by:
- using a male or female condom during sex
- being tested for HIV and sexually transmitted infections
- having a voluntary medical male circumcision
- using harm reduction services for people who inject and use drugs.
Doctors may suggest medicines and medical devices to help prevent HIV infection, including:
- antiretroviral drugs (ARVs), including oral Pre-Exposure Prophylaxis (PrEP) and long acting products
- dapivirine vaginal rings
- injectable long acting cabotegravir.
ARVs can also be used to prevent mothers from passing HIV to their children.
People taking antiretroviral therapy (ART) and who have no evidence of virus in the blood will not pass HIV to their sexual partners. Access to testing and ART is an important part of preventing HIV.
Antiretroviral drugs given to people without HIV can prevent infection
When given before possible exposures to HIV it is called pre-exposure prophylaxis (PrEP) and when given after an exposure it is called post-exposure prophylaxis (PEP). People can use PrEP or PEP when the risk of contracting HIV is high; people should seek advice from a clinician when thinking about using PrEP or PEP.
There is no cure for HIV infection. It is treated with antiretroviral drugs, which stop the virus from replicating in the body.
Current antiretroviral therapy (ART) does not cure HIV infection but allows a person’s immune system to get stronger. This helps them to fight other infections.
Currently, ART must be taken every day for the rest of a person’s life.
ART lowers the amount of the virus in a person’s body. This stops symptoms and allows people to live full and healthy lives. People living with HIV who are taking ART and who have no evidence of virus in the blood will not spread the virus to their sexual partners.
Pregnant women with HIV should have access to, and take, ART as soon as possible. This protects the health of the mother and will help prevent HIV transmission to the fetus before birth, or through breast milk.
Advanced HIV disease remains a persistent problem in the HIV response. WHO is supporting countries to implement the advanced HIV disease package of care to reduce illness and death. Newer HIV medicines and short course treatments for opportunistic infections like cryptococcal meningitis are being developed that may change the way people take ART and prevention medicines, including access to injectable formulations, in the future.
More information on HIV treatments
WHO response
Global health sector strategies on HIV, viral hepatitis, and sexually transmitted infections for the period 2022–2030 ( GHSSs ) guide strategic responses to achieve the goals of ending AIDS, viral hepatitis B and C, and sexually transmitted infections by 2030.
WHO’s Global HIV, Hepatitis and STIs Programmes recommend shared and disease-specific country actions supported by WHO and partners. They consider the epidemiological, technological, and contextual shifts of previous years, foster learning, and create opportunities to leverage innovation and new knowledge.
WHO’s programmes call to reach the people most affected and most at risk for each disease, and to address inequities. Under a framework of universal health coverage and primary health care, WHO’s programmes contribute to achieving the goals of the 2030 Agenda for Sustainable Development.
- Global HIV, Hepatitis and STIs Programmes
- Global Health Sector Strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030 (GHSS)
- GHSS report on progress and gaps 2024
- HIV country profiles
- HIV statistics, globally and by WHO region, 2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

A Review of Recent HIV Prevention Interventions and Future Considerations for Nursing Science
Author Contributions
As our knowledge of HIV evolved over the decades, so have the approaches taken to prevent its transmission. Public health scholars and practitioners have engaged in four key strategies for HIV prevention: behavioral-, technological-, biomedical-, and structural/community-level interventions. We reviewed recent literature in these areas to provide an overview of current advances in HIV prevention science in the United States. Building on classical approaches, current HIV prevention models leverage intimate partners, families, social media, emerging technologies, medication therapy, and policy modifications to effect change. Although much progress has been made, additional work is needed to achieve the national goal of ending the HIV epidemic by 2030. Nurses are in a prime position to advance HIV prevention science in partnership with transdisciplinary experts from other fields (e.g., psychology, informatics, and social work). Future considerations for nursing science include leveraging transdisciplinary collaborations and consider social and structural challenges for individual-level interventions.
Approximately 1.2 million people in the United States are currently living with HIV, and an estimated 14% are infected, yet unaware of their status ( Office of Infectious Disease and HIV/AIDS Policy, 2020 ). HIV and AIDS continue to have a disproportionate impact on certain populations, including youth—gay, bisexual, and other men who have sex with men (MSM)—racial and ethnic minorities, people who inject drugs, and residents of highly affected geographic regions such as the Southeastern United States ( Aral et al., 2020 ; Hill et al., 2018 ; Lanier & Sutton, 2013 ). Ending the HIV epidemic requires increasing engagement along the HIV prevention and care continua ( Kay et al., 2016 ; McNairy & El-Sadr, 2014 ). Early and repeat HIV testing are recommended strategies for early entry into HIV care and improved HIV-related outcomes ( DiNenno et al., 2017 ). Late and infrequent HIV testing may result in receiving an initial HIV diagnosis late in the disease trajectory, and individuals unaware they are living with HIV may be more likely to transmit HIV to others. It is essential for people living with HIV (PLWH) to receive a timely diagnosis so that they can begin combination antiretroviral therapy with the goal of achieving an undetectable viral load, a key HIV prevention strategy ( Cohen et al., 2016 ). To improve linkage and retention along the prevention and care continua, researchers have developed HIV prevention interventions in four key areas: behavioral-, technological-, biomedical-, and structural/community-level interventions.
Behavioral interventions are approaches that promote protective or risk-reduction behavior in individuals and social groups via informational, motivational, skill-building, and community-normative strategies ( Coates et al., 2008 ). HIV-specific examples include, but are not limited to, interventions promoting abstinence, condom use, sex communication, condom negotiation, HIV testing, reduction in number of sexual partners, stigma reduction, and use of clean needles among people who inject drugs. Behavioral interventions target various HIV risk behavior mediators and moderators, including symptoms of mental illness and emotion regulation ( Brawner et al., 2019 ), attitudes, beliefs, subjective norms, intentions ( Fishbein & Ajzen, 1975 ), and self-efficacy ( Bandura, 2001 ). The effectiveness of behavior-change interventions in reducing risk for HIV during the early stages of the HIV pandemic demonstrated the importance and utility of this approach ( Bekker et al., 2012 ). Classic approaches to behavioral interventions established in the early 1990s and 2000s paved the way for future research to build on, replicate, adapt, tailor, and disseminate a multitude of programs to meet the needs of various populations, such as women ( Jemmott et al., 2007 ; Wingood et al., 2004 ), MSM ( Crosby et al., 2009 ), and adolescents ( DiClemente et al., 2009 ; Jemmott et al, 1992 , 1998 , 1999 , 2010 ; Villarruel et al., 2007 ). To extend the reach of behavioral interventions, in recent years, researchers have begun leveraging technologies to promote protective and risk reduction behaviors.
The rapid expansion of the internet, mobile, and social computing technologies (e.g., text messaging, e-mail, chat, mobile phones, social media, video games, and geospatial networking applications) has provided new strategies for engaging populations who may be harder to reach through traditional venue-based HIV prevention interventions. Electronic health (eHealth) and mobile health (mHealth) have become especially popular for the delivery of technology-enabled HIV prevention interventions among populations reporting high technology ownership and use ( Barry et al., 2018 ; Conserve et al., 2016 ; Duarte et al., 2019 ; Henny et al., 2018; Hightow-Weidman & Bauermeister, 2020 ; Jongbloed et al., 2015 ; Maloney et al., 2020 ; Nadarzynski et al., 2017 ). In recent years, gamification, serious games, and virtual reality have been used in HIV prevention interventions to deliver highly engaged content and bolster interactions/behaviors in and outside of planned interventions ( Enah et al., 2013 ; Hightow-Weidman et al., 2017 , 2018 ; Liran et al., 2019 ; Muessig et al., 2015 , 2018 ). The potential role that technologies can play in increasing the scale of HIV prevention interventions, including those that aim to increase the adoption of biomedical HIV prevention methods, add to the appeal of these strategies ( Hightow-Weidman et al., 2020 ; Horvath et al., 2020 ; Maloney et al., 2020 ; Marcus et al., 2019 ; Muessig et al., 2015 ; Ramos et al., 2019 ; Threats & Bond 2021 ).
Efficacious biomedical advancements in HIV prevention, such as treatment as prevention (TasP) and pre-exposure prophylaxis (PrEP), remain underutilized because of structural barriers and social determinants of health ( Cahill et al., 2017 ; Jaiswal et al., 2018 ; Kuhns et al., 2019 ). TasP, postexposure prophylaxis (PEP), PrEP, and pharmacologic therapy for substance abuse treatment have proven to be effective for reducing the transmission of HIV and minimizing the risk of new HIV infection ( Coffin et al., 2015 ; El-Bassel & Strathdee, 2015 ; Hosek, Green, et al., 2013 ; Page et al., 2015 ; Springer et al., 2018 ). Although biomedical prevention methods such as PEP and TasP are highly effective, we explicitly focus on PrEP because of its status as the premiere user-controlled HIV prevention medication regimen and its ability to be taken without disclosure to sexual partner(s).
In addition to behavioral, technological, and biomedical strategies that help to mitigate risk at the individual-, structural-, and community-levels, additional intervention strategies are needed to address broader social and structural factors that contribute to inequitable geobehavioral vulnerability to HIV ( Brawner, 2014 ). Such HIV prevention interventions target contextual factors (e.g., social, political–economic, policy–legal, and cultural factors) that influence transmission of and infection with HIV ( Blankenship et al., 2015 ), with an effect that diffuses out to members of key populations. Where individual-level interventions are designed to change individual beliefs, norms, and behaviors, community-level interventions aim to change the social environment (e.g., community-level norms and collective self-efficacy) and behaviors of entire populations ( Underwood et al., 2014 ). Structural- and community-level interventions are crucial to a comprehensive HIV prevention plan because they are designed to target macrolevel contextual factors (e.g., concentrated disadvantage in neighborhoods, syringe exchange policies, community-level stigma, and limited PrEP accessibility) that shape individual risk or hamper adoption of risk reduction strategies and therapeutics ( Allen et al., 2016 ; Colarossi et al., 2016 ; Gamble et al., 2017 ; Hoth et al., 2019 ; Kerr et al., 2015 ). Researchers in this space have successfully partnered with churches to decrease stigma and increase HIV testing ( Berkley-Patton et al., 2016 ; Payne-Foster et al., 2018 ), expand access to screening and prevention resources and change provider behavior ( Bagchi, 2020 ; Bernstein et al., 2017 ; Wood et al., 2018 ), and increase HIV testing in correctional facilities ( Belenko et al., 2017 ).
In this review of the literature, we explore the evolution of HIV prevention science over the past 5 years, presenting an overview of recent advances in the United States. We focused on four intervention categories—behavioral-, technological-, biomedical- (PrEP), and structural-/community-level—given the advancement of HIV prevention approaches over time. The findings highlight areas where nurses and others can advance the science of strategies to reach the national goal of ending the HIV epidemic by 2030 ( Fauci et al., 2019 ).
In our review of recent randomized controlled trials testing the efficacy of condom use and/or abstinence interventions, most targeted vulnerable populations included MSM ( Arnold et al., 2019 ; Crosby et al., 2018 ; Rhodes et al., 2017 ), adolescents ( Donenberg et al., 2018 ; Houck et al., 2016 ; Peskin et al., 2019 ), and adolescent/caregiver dyads ( Hadley et al., 2016 ; Jemmott et al., 2019 , 2020 ). Other interventions targeted incarcerated women ( Fogel et al., 2015 ) and illicit drug users ( Tobin et al., 2017 ). Many interventions were delivered in a health care or school setting, where multiple 60- to 120-min group sessions were presented. However, some interventions used a variation of frequencies and durations, such as a single 60-min one-to-one session ( Crosby et al., 2018 ) or multiple 4-hour group sessions ( Rhodes et al., 2017 ). One intervention allowed participants to attend one or two independent learning sessions, totaling 3 hours ( Hadley et al., 2016 ). About half of the reviewed studies tested the efficacy of new interventions, whereas the other half adapted previously established behavioral interventions. Crosby et al. (2018) , Fogel et al. (2015) , and Hadley et al. (2016) each adapted a different sexual health intervention. Conversely, Donenberg et al. (2018) adapted an intervention from a combination of three evidence-based programs. Instead of adapting an intervention, Peskin et al. (2019) replicated an evidence-based intervention. All interventions, except one ( Rhodes et al., 2017 ), were delivered in English.
Although several behavioral interventions led to significant increases in condom use and/or abstinence, compared with control conditions, others found no indication of intervention efficacy. For instance, Hadley et al. (2016) reported no significant group by time differences for condom use at the 3-month follow-up. These findings may be the result of delayed effects, as seen in the Fogel et al. (2015) intervention where significant group differences in condom use occurred at the 6-month follow-up, but not at the 3-month follow-up. Conversely, neither Arnold et al. (2019) nor Donenberg et al. (2018) found significant group by time differences in condom use at or beyond the 6-month follow-up, and Peskin et al. (2019) did not find differences in sexual initiation at the 24-month follow-up. These findings show that longer follow-up periods do not always have better results compared with short (i.e., 3 months) follow-up periods.
Among efficacious behavioral interventions, there was a single-session skill-building condom buffet activity ( Crosby et al., 2018 ) and multisession programs focusing on the costs and benefits of behavior change ( Fogel et al., 2015 ), emotion regulation ( Houck et al., 2016 ), cultural values ( Rhodes et al., 2017 ), environmental stressors ( Tobin et al., 2017 ), mother–son communication ( Jemmott et al., 2019 ), and scripture- and nonscripture-based abstinence ( Jemmott et al., 2020 ). Each was rooted in theoretical foundations, including those of the Theory of Planned Behavior, Social Cognitive Theory, cognitive behavioral techniques, and the AIDS Risk Reduction Model ( Azjen, 1991 ; Bandura, 1991 ; Catania et al., 1990 ). The Jemmott et al. (2019) intervention was unique in that intervention outcomes were assessed for both mothers and their sons, although the intervention was only implemented among the mothers. All other studies reported the findings of interventions that were implemented among the same participants for which outcomes were assessed.
We unexpectedly identified a nearly equal number of efficacious and nonefficacious behavioral interventions. Many studies reported increases in constructs leading to behavior change, such as self-efficacy, intentions, and attitudes, but did not find changes in actual condom use or abstinence behaviors ( Arnold et al., 2019 ; Donenberg et al., 2018 ; Hadley et al., 2016 ; Peskin et al., 2019 ). Interventions that were effective in increasing condom use and/or abstinence varied in characteristics, showing that single-session and multisession, short- and long-duration, newly created and adapted interventions can be efficacious.
Of the four interventions adapted from existing evidence-based programs, two were efficacious ( Crosby et al., 2018 ; Fogel et al., 2015 ). These interventions adapted the Focus on the Future ( Crosby et al., 2009 ) and Sexual Awareness for Everyone ( Shain et al., 1999 ) interventions. Adaptation has been long supported as an effective way to bring evidence-based interventions to new target populations and is often preferred over the creation of new interventions ( McKleroy et al., 2006 ; Solomon et al., 2006 ). However, with only half of the adapted interventions showing increased condom use among participants, it is critical that interventionists pay close attention to fidelity, proper use of theoretical foundations, and inclusion of core components during intervention adaptation and replication to maintain efficacy of the original intervention.
In accordance with previous research, the identified studies indicate the need to move away from interventions addressing a single behavior to those focusing on a combination of behavioral, biomedical, and structural approaches ( Belgrave & Abrams, 2016 ; Kurth et al., 2011 ) and those using popular technologically advanced delivery methods.
Technological Interventions
Most of the technological intervention literature we reviewed reported on studies that were at lower levels of the hierarchy of evidence ( Melnyk & Fineout-Overholt, 2011 ). Specifically, there was an imbalance in studies favoring more formative qualitative work or pilot studies compared with fewer studies reporting on randomized control trials or meta-analysis. Many studies reporting on randomized control trials also did not report on HIV-related outcomes but focused on content, protocols, or feasibility aspects of the randomized control trials. As a result, most studies reviewed were cross-sectional descriptive studies or qualitative descriptive studies using data collection strategies such as focus groups, interviews expert panels, surveys, and mixed methods ( Rodríguez Vargas et al., 2019 ; Velloza et al., 2019 ). Many of these formative studies ( Bauermeister et al., 2019 ; Cordova et al., 2018 ; Do et al., 2018 ; Enah et al., 2019 ; Erguera et al., 2019 ; Maloney et al., 2020 ; Sullivan et al., 2017 ) focused on the appeal, usability, acceptability, and feasibility of using mHealth and eHealth HIV prevention intervention across the continuum from primary prevention to disease management.
Reviewed studies show that technological interventions in HIV prevention have been studied in various target populations. These target populations include youth and young adults ( Cordova et al., 2018 ; Do et al., 2018 ; Erguera et al., 2019 ), MSM ( Alarcón Gutiérrez et al., 2018 ; Fan et al., 2020 ; Holloway et al., 2017 ), incarcerated women ( Kuo et al., 2019 ), couples ( Mitchell, 2015 ; Velloza et al., 2019 ), adults in drug recovery ( Liang et al., 2018 ), and PLWH ( Bauermeister et al., 2018 ; Schnall et al., 2019 ). These interventions vary in the technological innovations used, duration, language, format of delivery, and target outcomes.
The technology-enabled intervention studies reviewed used varying strategies to target different areas along the continuum of HIV prevention and care. In HIV testing, the focus was on synching home test results with phone counseling support ( Wray et al., 2017 ) and ordering, scheduling, and reminders associated with testing kits (e.g., Sullivan et al., 2017 ). Some studies focused on education using interactive web-based games and social media platforms ( Bauermeister et al., 2019 ; Bond & Ramos, 2019 ; Cordova et al., 2018 ; Gabarron & Wynn, 2016 ), behavioral change interventions ( Danielsonetal.,2014 ),or linkage to care and care support ( Bauermeister et al., 2018 ) targeting people who are living without HIV. Other studies focused on similar points along the care and prevention continuum targeting PLWH ( Maloney et al., 2020 ). Studies reviewed also focused on a wide range of interventions such as provision of information, self-assessments, adherence reminders, delivery of prevention information, referrals, and service from providers ( Boni et al., 2018 ; De Boni et al., 2018 ; Maloney et al., 2020 ). A number of studies included text messaging for health education, reminders, and assessments ( Dietrich et al., 2018 ; Njuguna et al., 2016 ; Ware et al., 2016 ), whereas others focused on primary behavioral preventions such as drug use ( Cordova et al., 2018 ) and engagement in care and disease management for PLWH ( Fan et al., 2020 ; Jongbloed et al., 2015 ). Reviewed studies targeting both persons living with and without HIV also used various technological-based approaches, such as interactive web-based content ( Bauermeister et al., 2019 ), smartphone geolocators near gay venues reinforcing safer sex practices ( Besoain et al., 2015 ), immersive adventure games ( Enah et al., 2019 ), and use of eye tracking technologies to monitor use ( Cho et al., 2018 , 2019 ).
Eight studies reviewed were narrative, scoping, and systematic reviews of the use and efficacy of technology-based interventions ( Bailey et al., 2015 ; Duarte et al., 2019 ; Gabarron & Wynn, 2016 ; Henny et al., 2018; Jongbloed et al., 2015 ; Maloney et al., 2020 ; Nadarzynski et al., 2017 ; Niakan et al., 2017 ). Scoping reviews of earlier digital STI prevention interventions revealed moderate effects on sexual health knowledge, small effect of behavior change, and no significant changes in biological outcomes ( Bailey et al., 2015 ; Gabarron & Wynn, 2016 ). These reviews examined studies that incorporated interventions using various designs, content, formats, target populations, and quality of content. Taken together, these reviews suggest that more research is needed to identify or develop components that can promote changes in biological outcomes. The most recent systematic review ( Maloney et al., 2020 ) found a wealth of published literature on technology-based interventions. However, findings from this systematic review suggest that most of the studies focus on educational and behavior change interventions, whereas relatively few focused on linkage to and retention in HIV prevention and care and adherence to HIV medicines, especially PrEP.
The drug combination of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC), widely known by its brand name Truvada, used as oral PrEP in preventing HIV infections for MSM in the United States, is a well-documented, effective prevention strategy ( Grant et al., 2010 ; Mayer et al., 2020 ). However, many communities impacted by HIV are underrepresented in research trials in the United States, including transgender populations, cisgender women, and people who inject drugs. Most of the research in these groups has occurred internationally and has not had as strong an impact on HIV incidence as that of the MSM ( Baeten et al., 2012 ; Kibengo et al., 2013 ; MacLachlan & Cowie, 2015 ; Marrazzo et al., 2015 ; Martin et al., 2017 ; Mutua et al., 2012 ; Peterson et al., 2007 ; Thigpen et al., 2012 ; Van Damme et al., 2012 ).
Despite Black MSM bearing a disproportionate burden of HIV infection in comparison to MSM of other ethnicities, they are underrepresented in PrEP studies ( Hess et al., 2017 ). Most PrEP clinical trials, open-label studies, and observational studies included less than 10% Black MSM. ( Grant et al., 2010 ; Mayer et al., 2020 ). The few studies that included higher proportions of Black MSM had small numbers, including three community studies by Chan (49%, n = 109), Project PrEPare-ATN 082 (53%, n = 31), Project PrEPare-ATN 110 (47%, n = 93), and Project PrEPare-ATN 113 (29%, n = 23; Hosek et al., 2017 ; Hosek, Siberry, et al., 2013 ). Moreover, there are study gaps in sex, and the number of studies on high-risk cisgender women and transgender women is significantly smaller compared with MSM ( Kamitani et al., 2019 )
In 2019, the U.S. Food and Drug Administration (FDA) approved tenofovir alafenamide/emtricitabine (TAF/FTC), widely known as Descovy, as the first alternative medication for PrEP for MSM and transfeminine communities (FDA, 2019). TAF/FTC was shown to be noninferior to TDF/FTC. The side-effect profiles differ in that TDF/FTC has increases in renal and bone toxicities and TAF/FTC has increases in weight and lipids ( Mayer et al., 2020 ). TAF/FTC was not studied in other communities and did not gain an FDA indication for cisgender women and transmasculine communities. Some studies have found a potential link between the use of estradiol for gender-affirming care and lower tenofovir levels in the blood ( Hiransuthikul et al., 2019 ; Shieh et al., 2019 ; Yager & Anderson, 2020 ).There have been smaller studies to verify this interaction, but reported controlling for confounding variables was difficult. Further research is needed to understand whether there is effect on the efficacy of TDF and how this may impact nondaily dosing strategies.
Currently, daily oral PrEP is the only antiretroviral medication recommended for the prevention of HIV through sexual contact and drug injection use among people without HIV by the U.S. Centers for Disease Control and Prevention (CDC; U.S. Centers for Disease Control and Prevention & U.S. Public Health Service, 2018 ). In 2015, Molina et al. (2015) published a placebo-controlled trial of an “On-Demand” or 2-1-1 dosing strategy for MSM and transfeminine communities where 2 TDF/FTC pills would be taken 2 to 24 hr before sex, followed by 1 pill every 24 hr while sex continues, and ending 2 doses after the last sex act. This study found high efficacy and acceptability with an 86% reduction in HIV incidence relative to placebo on an intention to treat basis; no one acquired HIV while using 2-1-1 dosing of this nondaily dosing strategy. Furthermore, additional prospective open-label studies also showed no HIV acquisition among study participants ( Hojilla et al., 2020 ; Siguier et al., 2019 ). Despite the lack of endorsement by the CDC, many local Departments of Public Health support PrEP 2-1-1 as a way to make PrEP more attainable for the MSM community ( Los Angeles County Department of Public Health, 2019 ; New York City Department of Health and Mental Hygiene, 2019 ; San Francisco Department of Public Health, 2019 ).
Although effective, researchers and primary care providers note the need to simplify current PrEP delivery models. The CDC recommends a follow-up visit every 3 months while on PrEP, which can often be challenging for individuals to attend visits and pay laboratory costs ( U.S. Centers for Disease Control and Prevention & U.S. Public Health Service, 2018 ). A new model of care that leverages mHealth (e.g., mobile and social computing technologies) to increase initiation, retention, and adherence to PrEP, such as electronic PrEP (ePrEP), has been introduced and found to be acceptable and effective among PrEP users ( Siegler et al., 2020 ). PrEPmate is one such multicomponent mHealth intervention that uses short-message service (SMS) and youth-tailored interactive online content to enhance PrEP adherence among at-risk young MSM ( Liu et al., 2019 ). Currently, it is the only PrEP study identified as an Evidence-Based Intervention by the CDC Prevention Research Synthesis project ( CDC, 2020 ). Siegler et al. implemented the PrEP at Home Study among 50 young Black MSM in a rural area. In the study, 42% of participants received PrEP via the ePrEP system, whereas 93% preferred to use ePrEP over standard provider visit and 67% were more likely to remain on PrEP if ePrEP were available ( Siegler et al., 2019 ).
Future biomedical HIV prevention modalities such as long-acting injectable agents have the potential to prevent HIV acquisition without relying on adherence to a daily or 2-1-1 oral dosing regimen. In MSM and transfeminine communities, an injectable form of cabotegravir given intramuscularly every 2 months had an estimated 66% lower incidence of HIV, compared with daily TDF/FTC ( Landovitz, 2020 ). Additional cabotegravir studies in cisgender women are being conducted under HPTN 084 to evaluate safety and efficacy (the LIFE Study; HIV Prevention Trials Network, 2020 ). The dapivirine (DAP) vaginal ring, for use by cis-women as a flexible silicone ring that continuously releases the antiretroviral HIV drug DAP in the vagina as a long-acting option for HIV prevention is another biomedical HIV prevention modality being studied ( Psomas et al., 2017 ). A phase 2a trial of a 25-mg DAP vaginal ring has been shown to be safe and acceptable among U.S. adolescents ages 15–17 ( Psomas et al., 2017 ). The DAP vaginal ring has been approved by the European Medicines Agency for women older than 25 years, and further studies are ongoing for women ages 15–25 years in the United States ( National Institutes of Health, 2020 ).
Although there are clear benefits to the aforementioned intervention strategies, structural- and community-level interventions are distinctly different, given their focus on macrolevel factors that influence risk versus individual beliefs and behaviors. This is imperative because in many highly affected demographics (e.g., Black women and young racial and ethnic minority MSM), broader social and structural factors drive HIV risk more than individual behavior ( Bauermeister et al., 2017 ; Brawner, 2014 ). With this wider focus, changes are seen in factors such as social diffusion of safer sex messages and comfort with being gay ( Eke et al., 2019 ), better viral suppression and continuity in care ( El-Sadr et al., 2017 ; Towe et al., 2019 ; Wohl et al., 2017 ), and increased HIV testing in populations that may not have otherwise been tested ( Belenko et al., 2017 ; Berkley-Patton et al., 2019 ; Frye et al., 2019 ).
Addressing structural barriers can reduce viral load, prevent HIV infection, and increase HIV testing. In homeless populations, researchers used a rapid rehousing intervention to place participants in stable housing faster (3 months earlier than usual service clients), doubling the likelihood of achieving or maintaining viral suppression ( Towe et al., 2019 ), and worked through primary care providers in Veterans Affairs to increase PrEP access ( Gregg et al., 2020 ). Community-level interventions that used financial incentives reduced viral load and decreased self-reported stimulant use among sexual minority men who use methamphetamine ( Carrico et al., 2016 ) and increased viral suppression and continuity in care in HIV-positive patients ( El-Sadr et al., 2017 ). The latter intervention, however, did not demonstrate an effect on increasing linkage to care.
Health care access remains a concern, and novel strategies can be used to get services to those in need. Pharmacies have also been promising locations for HIV prevention work. Persons who inject drugs were more likely to report always using a sterile syringe than not when they were connected to pharmacies that received in-depth harm reduction training and provided additional services (i.e., HIV prevention/medical/social service referrals and syringe disposal containers; Lewis et al., 2015 ). Providing a PEP informational video and direct pharmacy access to PEP also increased PEP knowledge and willingness; however, this did not translate to more PEP requests ( Lewis et al., 2020 ).
In correctional facilities, researchers have used strategies such as referral to care within 5 days after release, medication text reminders, and local change teams with external coaching to maintain viral suppression post-release and increase HIV testing among inmates ( Belenko et al., 2017 ; Wohl et al., 2017 ). High fidelity to the required institutional changes needed to improve HIV services was also noted ( Pankow et al., 2017 ). With the detrimental effects of mass incarceration, including disparate HIV outcomes while incarcerated and post-release, correctional settings are prime targets for future structural intervention work.
Success is tied to meeting people where they are—engaging them through existing programs, organizations, and institutions they are already connected to. Congregation-level interventions have demonstrated success in doubling HIV testing rates and reducing HIV stigma ( Berkley-Patton et al., 2019 ; Derose et al., 2016 ; Payne-Foster et al., 2018 ); however, effects on HIV stigma varied across studies. The studies demonstrating an effect on HIV stigma only achieved this at the individual—not congregation—level ( Payne-Foster et al., 2018 ), and in Latino—but not African American—churches ( Derose et al., 2016 ). Key to these interventions was the inclusion of multilevel activities (e.g., ministry group activities, HIV testing events during services, and pastors delivered sermons on HIV-related topics) and flexibility to accommodate church schedules and levels of comfort with covering different topics. Churches were not the only setting where addressing HIV stigma beyond the individual-level was a challenge. In a community-level intervention on HIV stigma, homophobia, and HIV testing, researchers used workshops, space-based events, and bus shelter ads in a high HIV prevalence area but did not have an effect on HIV stigma or homophobia ( Frye et al., 2019 ). They did, however, increase HIV testing by 350%.
Individuals within key systems and communities can also be pivotal to share HIV-related information and increase access to services. Integration of lay health advisors (“Navegantes”) into existing social networks (i.e., recreational soccer teams) among Hispanic/Latino men led to twice the likelihood of reporting consistent condom use in the past 30 days and HIV testing at the 18-month follow-up ( Rhodes, Leichliter, et al., 2016 ). A year after the intervention ended, 2 years after their training, 84% of the Navegantes (16 of 19) continued to conduct 9 of the 10 primary health promotion activities (e.g., talking about sexual health, describing where to get condoms, and showing segments of the intervention DVD; Sun et al., 2015 ). Furthermore, using a popular opinion leader model targeting alcohol-using social networks, researchers demonstrated a decline in composite sexual risk (e.g., having sex while high or with a partner who is high and exchanging sex for drugs or money) and an increase in HIV knowledge ( Theall et al., 2015 ). An intervention developed for college students and those in the surrounding area integrated HIV testing and education, mental health, and substance abuse services and referrals and noted a preliminary effect on social norms and sexual health messages on campus ( Ali et al., 2017 ).
Culturally situated marketing and other media approaches reach a broader audience to effect change. Successful social marketing campaigns to promote HIV testing should be performed in a way that enhances well-being (rather than fear-based messages), does not represent the target community in stigmatizing ways, and acknowledges barriers to HIV testing (e.g., stigma; Colarossi et al., 2016 ). One study evaluated a city-level, culturally-tailored media intervention combined with an individual risk reduction curriculum in comparison to no city-level media and a general health curriculum ( Kerr et al., 2015 ). Study findings suggested that all media-exposed participants had greater HIV-related knowledge at 6 months, and those who received the media intervention and risk reduction content had lower stigma scores at 3 and 12 months. A community-level intervention designed to decrease HIV risk among young MSM via persuasive media communication and peer-led networking outreach reduced anal sex risk among participants who reported binge drinking and/or marijuana use; the effect was not sustained for those who used other drugs ( Lauby et al., 2017 ). Another community mobilization intervention (e.g., publicity, groups, and outreach) addressed psychosocial factors at individual, interpersonal, social, and structural levels and documented an increase in HIV testing and a reduction in condom-less sex (although not sustained at 6 months; Shelley et al., 2017 ).
Interventions targeting providers and care delivery increase risk screening, HIV testing, timely linkage to care, and PrEP access for eligible individuals. Similar to the ways lay health workers are activated internationally, Health Promotion Advocates were employed in pediatric emergency departments to survey patients (e.g., health risks, stresses, and needs; Bernstein et al., 2017 ). Positive screens triggered critical resources (e.g., brief conversation on risks and needs and treatment as indicated), and, as a result, the intervention extended emergency services beyond the scope of the presenting complaint, engaging more than 800 youth in critical services such as mental health treatment and HIV testing. By pairing intensive medical case management with formalized relationships with local health departments and resources and addressing structural barriers (e.g., ability to access HIV prevention, testing, and medical care), researchers were able to decrease the average number of days to link to care and maintain the decline over a 6-year period ( Miller et al., 2019 ). Ninety percent of those linked to care had an initial medical visit in 42 or fewer days postdiagnosis. The integration of PrEP referrals into STI partner services led to 54% of PrEP eligible men accepting a PrEP referral and a 2.5-fold increase in PrEP use after partner services among MSM ( Katz et al., 2019 ).
Another group had health professional students (e.g., medicine and pharmacy) provide education about PrEP to public health providers, contributing to an increase in PrEP prescriptions, including for PrEP-eligible at-risk groups who previously were not given prescriptions ( Bunting et al., 2020 ). An underway pilot targets training primary care providers to better understand historical influences of structural factors, assess structural vulnerability among patients, create a more integrated system of care (e.g., opioid use and HIV risk) and empathy and nonjudgement in patient interactions ( Bagchi, 2020 ). There is strong precedent for this, given that significant effects were noted in creating affirming environments for sexual and sex minority youth, including improvements in providers’ and staff’s knowledge and attitudes, clinical practices, individual practices, and perceived environmental friendliness/safety ( Jadwin-Cakmak et al., 2020 ).
Policy changes can hinder or advance HIV prevention efforts, and modeling is an effective strategy to project outcomes and identify targeted prevention strategies. In an examination of Washington, DC’s buffer zone policy—prohibition of syringe exchange program operations within 1,000 feet of schools—researchers found that adherence to this 1,000 Foot Rule reduced syringe exchange program operational space by more than 50% a year ( Allen et al., 2016 ). These restrictions on the amount of legal syringe exchange program operational space have a significant impact on service delivery among injection drug users, which in turn affects HIV transmission through syringe sharing ( Allen et al., 2016 ). Analysis of a natural policy intervention indicated that removing a ban that prohibited the use of federal funds for syringe exchange programs potentially averted 120 HIV cases ( Ruiz et al., 2016 ).
In examining which prevention approach would achieve the greatest impact on HIV transmission, in light of available resources, study findings suggested that targeted testing by venue is more cost effective than routine emergency department testing ($31,507 vs. $59,435, respectively; Holtgrave et al., 2016 ). Modeling of interventions in 6 cities indicated that HIV incidence could be reduced by up to 50% by 2030, with cost savings of $95,416 per quality-adjusted life-year, by implementing combinations of evidence-based interventions (e.g., medication for opioid use disorder, HIV testing, ART initiation, and retention; Nosyk et al., 2020 ). Of note, nurse-initiated rapid testing was included in the optimal combination that produced that greatest health benefit while remaining cost effective across all cities. An ongoing microenterprise RCT will determine the effects of multiple strategies (e.g., weekly text on job openings, educational sessions on HIV prevention, and $11,000 start-up grant) on sexual risk behaviors, employment, and HIV preventive behaviors among economically vulnerable African American young adults ( Mayo-Wilson et al., 2019 ); a paucity of reviewed studies focus in this area. A comparable holistic health demonstration project, which engaged young Black MSM, successfully achieved viral suppression, connected participants to employment opportunities, and addressed housing discrimination ( Brewer et al., 2019 ).
Discussion and Future Considerations for Nursing Science
This review of current HIV prevention interventions provides a substantial contribution to the literature by synthesizing literature on four key areas of HIV prevention science. Nursing focuses on holistic care, assessing, diagnosing, and treating all areas that influence individual and population health. As we consider where and how to develop these programs, research indicates that more people may receive HIV prevention interventions in community-based clinics than in primary care or acute care settings ( Levy et al., 2016 ). Future nursing research should aim to address the needs of underserved populations who may benefit from robust HIV prevention strategies as outlined in this discussion section.
As we continue to generate knowledge about the multidimensional nature of HIV risk, especially for marginalized and vulnerable populations, there are increasing opportunities to learn from and use previous research to design multilevel and combination intervention strategies to better overcome barriers to HIV prevention ( Brawner, 2014 ; Frew et al., 2016 ). As suggested by the identified behavioral intervention studies, classic and current prevention programs have used useful strategies, but there remains room for improvement. These studies advance the science of HIV prevention, which helps fill gaps in the current literature and offer valuable insights that can contribute toward advancing the plan of Ending the HIV Epidemic ( U.S. Department of Health and Human Services, 2019 ; Treston, 2019 ).
As behavioral interventions continue to be created, replicated, and adapted, researchers should focus on implementing and testing these interventions in real-life settings. Implementation science strategies include planning, education, finance, restructuring, quality management, and policy strategies ( Powell et al., 2012 ). These strategies include various aspects of collecting data from stakeholders and community members, assessing setting readiness, determining realistic dosing, and assessing intervention acceptability and feasibility among target populations. The translation of science from research settings to real-life settings is imperative in the sustainability of efficacious behavioral interventions.
Technological
Although there is a plethora of technological-based HIV interventions with many in the pipeline, gaps persist in the current literature. There is a lack of precise knowledge regarding the content components of these interventions that are associated with improving clinical outcomes ( Dillingham et al., 2018 ; Ramos, 2017 ). There is also limited knowledge of optimal delivery approaches for these types of digital HIV interventions ( Côté et al., 2015 ; Schnall et al., 2015 ). In addition, there is a dearth of studies evaluating the efficacy, effectiveness, and cost effectiveness of using emerging technologies in HIV prevention interventions, such as gaming, gamification, social media, and virtual interventions ( Garett et al., 2016 ; Kemp & Velloza, 2018 ; LeGrand et al., 2018 ). Furthermore, there is a lack of resource-sharing platforms that would allow for new research to build on impactful elements of technology-based HIV prevention interventions without recreation of these components. Making these components available in an open platform would substantially reduce time and costs of developing new technological interventions and prevent wasteful use of resources on elements that do lead to desired outcomes.
In all, because technology continues to evolve and potential users of these interventions gain more access and complex skills in the use of other applications in everyday life, the demand for more user-centric HIV prevention interventions will likely continue to grow. Current interventions will need to be updated to maintain relevance, and new interventions will need to be designed to be adaptable to continuing technological advances. Policymakers have a role to play in allowing for governmental sharable databases of impactful interventions so that limited resources can be used to design predictably effective components of technological interventions leading to better health outcomes.
The nurse plays a vital role in HIV prevention and PrEP care ( O’Byrne et al., 2014 ). The University of California, San Francisco School of Nursing, recently developed and validated a set of entry-level nurse practioner competencies to provide culturally appropriate comprehensive HIV care ( Portillo et al., 2016 ). Similar programs should be implemented to train nurses and further the delivery of nursing-led biomedical HIV interventions. Magnet is a nurse-led clinic in San Francisco that has successfully leveraged expanded scopes of practice to allow for nurses to practice to the full extent of their licensure and allow for the rapid expansion of PrEP services to the community ( Holjilla et al., 2018 ). Such a unique and successful community-based PrEP delivery intervention led to the development and implementation of pharmacist-led PrEP clinics ( Havens et al., 2019 ; Lopez et al., 2020 ; Tung et al., 2018 ). More community-based, nursing-led biomedical HIV interventions are needed. Furthermore, future research should explore the efficacy of biomedical HIV prevention among transmasculine and cis-women populations, especially those of color who are underrepresented in existing research efforts ( Bond & Gunn, 2016 ; Chandler et al., 2020 ; Deutsch et al., 2015 ; Golub et al., 2019 ; Rowniak et al., 2017 ; Willie et al., 2017 ). Community-based nursing-led HIV interventions may be opportune for reaching these populations.
Structural and Community
Most HIV prevention structural- and community-level interventions still focus on developing countries, with less attention in the United States ( Adimora & Auerbach, 2010 ). However, with several communities facing limited resources, large percentages of individuals living below the federal poverty level and high HIV incidence and prevalence rates, it is time to expand these international success stories to domestic work (e.g., microfinance, credit programs, and comprehensive sexual health education). There is also a paucity of these interventions targeted to women and youth. Relative to the other reviewed strategies, very few nurses are engaged in structural-/community-level interventions. If successful, the in-progress microenterprise RCT by Mayo-Wilson et al. (2019) has the potential to serve as a blueprint for integrating multiple structural approaches that have demonstrated effectiveness abroad into U.S. contexts. The work by Werb et al. (2016) can also transform approaches to structural approaches to prevent injection drug initiation—given nursing’s focus on prevention, initiation of, and/or partnership in such work could be pivotal.
An approach to consider moving forward is applying the multiphase optimization strategy (MOST) to HIV prevention strategies ( Collins et al., 2016 ). MOST uses randomized experimentation to assess the individual performance of each intervention component. This rigorous process, based on a priori optimization criteria (e.g., cost and time), identifies whether aspects of an intervention component (e.g., presence, absence, and setting) have an impact on the performance of other components. Ultimately, this knowledge is used to engineer an intervention that is effective, efficient, and readily scalable. Multilevel interventions that target more than one level can lead to the most sustainable behavior change and can be delivered in venues known to be associated with HIV risk (e.g., bars and nightclubs; Pitpitan & Kalichman, 2016 ). An ongoing study on neighborhood contexts (e.g., poverty, HIV prevalence, and access to care) and network characteristics (e.g., size and frequency of communication) among Black MSM in the deep south will generate rich data to inform interventions for this key demographic ( Duncan et al., 2019 ). There also remains a need for explicit research with transgender populations—versus only including them in other samples—to fill gaps and meet unique needs ( Mayer et al., 2016 ).
Nursing Advancements
Nurse scientists around the globe are contributing to the development of interventions along the care continuum. Jemmott et al. have created numerous behavioral interventions over the past 30 years, which have been adapted for use in new settings and with different populations ( Advancing Health Equity: ETR, 2019 ). Behavioral interventions have been implemented using technology. Nursing exemplars in technology include the development of an immersive adventure game for African American adolescents ( Enah et al., 2019 ; Enahet al., 2015). In a series of studies in the primary prevention end of the prevention and care continuum, Enah et al. studied relevant content and design elements, evaluated an existing web-based game for relevance, developed and qualitatively studied acceptability of an individually tailored adventure game, and evaluated the potential efficacy of the game among African American adolescents ( Enah et al., 2019 ; Enah, Piper & Moneyham, 2015 ). At the care end of the continuum, Schnall et al. adapted an existing intervention for MSM ( Schnall et al., 2016 ), addressing co-morbidities for PLWH using multimodal techniques ( Schnall et al., 2019 ).
Flores developed a novel sex communication video series to support parent–child communication for gay, bisexual, and queer male adolescents ( Flores et al., 2020 ). Nurses have also collaborated on telehealth interventions to identify barriers to HIV care access and adherence and address mental health, substance use, and other issues among youth and young adults living with HIV ( Wootton et al., 2019 ). Other researchers piloted HIV/STI prevention content curated from online resources (e.g., YouTube and public and private websites) and found that youth who received links to publicly accessible online prevention content had a significant improvement in HIV self-efficacy and a significant reduction in unprotected vaginal or anal sex ( Whiteley et al., 2018 ). Nurses can partner with public health experts, computer scientists, and others to leverage these resources for population health improvement because new technologies continue to emerge ( Rhodes, McCoy, et al., 2016 ; Stevens et al., 2017 ; Stevens et al., 2020 ). Nurses, including members of the Association of Nurses in AIDS Care, have also been instrumental in the All of Us Research Program, a National Institutes of Health initiative aiming to enroll one million people across the United States to increase accessibility to data on individual variability in factors including genetics, lifestyle, and socioeconomic determinants of health to speed up medical breakthroughs ( National Institutes of Health, 2020 ).
As experts in community-engaged HIV research, partnerships that are committed to engaging key communities will lead to the development of interventions—across levels—to help achieve national goals of ending the HIV epidemic by 2030 ( Fauci et al., 2019 ). Health departments, academia, and community partners can also collaborate on policy modeling to improve resource allocation and better address HIV prevention priority setting ( Holtgrave et al., 2016 ). Investigators have also called for legal reform to address state-level structural stigma (index including density of same-sex couples and state laws protecting sexual minorities) experienced by MSM, given linkages between decreased state-level stigma and reduced condomless anal intercourse and increased PEP and/or PrEP use ( Oldenburg et al., 2015 ). Geographic information systems mapping can also be used to identify areas of greatest need and allocate necessary resources ( Brawner et al., 2017 ; Brawner, Reason, Goodman, Schensul, & Guthrie, 2015 ; Eberhart et al., 2015 ).
Recommendations to Further Advance HIV Prevention Intervention Science
Based on the literature reviewed and gaps identified, we offer three recommendations to further advance HIV prevention intervention science. First, nurses should leverage transdisciplinary partnerships to lead the development and testing of comprehensive interventions. For example, nurses could develop and test a nurse-delivered intervention model that engages pharmacists for PrEP access, psychologists and social workers for mental health treatment, librarians and health communication scholars for improving health literacy, and health informaticists to program the content for virtual delivery. Second, nurse researchers are at the cutting edge of knowledge generation in multiple fields, including HIV science (as evidenced by this review), and should be highly sought after for research collaboration accordingly. Although nursing is one of the most trusted professions, nurses are often overlooked when researchers in other disciplines search for collaborators with advanced methodological skill sets, content-specific expertise, or additional perceived benefits to their research teams. Finally, regardless of the intervention type (e.g., behavioral and biomedical), future intervention work must account for social and structural challenges experienced by the intended intervention recipients (e.g., racism, homelessness, and concentrated poverty in neighborhoods). This could include activities such as adding social service linkages to research protocols (e.g., providing participants with information on stable housing programs) or implementing structural interventions to improve neighborhood conditions (e.g., hiring community members to green vacant lots).
Nurses have made tremendous strides in behavioral interventions; however, representation in biomedical-, technological-, and structural-/community-level interventions is limited. We believe this hinders possible advancements in HIV prevention science, given the uniqueness a nursing lens contributes to research endeavors. We encourage nurses to expand the scope of their intervention work, and for individuals working in fields of HIV prevention where nurses are underrepresented, to seek out nursing collaborators. Together, these transdisciplinary teams can curb the epidemic and achieve an AIDS-free generation.
Key Considerations
- Nurses should leverage transdisciplinary partnerships to lead the development and testing of comprehensive interventions.
- Future intervention work must account for social and structural challenges experienced by the intended intervention recipients.
- Nurse researchers should be used for their advanced methodological skill sets and expertise in HIV prevention science.
Disclosures
The authors report no real or perceived vested interests related to this article that could be construed as a conflict of interest.
- Adimora AA, & Auerbach JD (2010). Structural interventions for HIV prevention in the United States . Journal of Acquired Immune Deficiency Syndromes , 55 ( Suppl 2 ), S132–S135. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Advancing Health Equity: ETR. (2019). Be Proud! Be Responsible! An Evidence-Based Intervention to Empower Youth to Reduce Their Risk of HIV . Retrieved from https://www.etr.org/ebi/programs/be-proud-be-responsible/
- Alarcón Gutiérrez M, Fernández Quevedo M, Martín Valle S, Jacques-Aviñó C, Díez David E, Caylà JA, & García de Olalla P (2018). Acceptability and effectiveness of using mobile applications to promote HIV and other STI testing among men who have sex with men in Barcelona , Spain. Sexually Transmitted Infections , 94 ( 6 ), 443–448. 10.1136/sextrans-2017-053348 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ali S, Al Rawwad T, Leal RM, Wilson MI, Mancillas A, Keo-Meier B, & Torres LR (2017). SMART cougars: Development and feasibility of a campus-based HIV prevention intervention . Journal of Health Care for the Poor and Underserved , 28 ( 2 ), 81–99. [ PubMed ] [ Google Scholar ]
- Allen ST, Ruiz MS, & Jones J (2016). Quantifying syringe exchange program operational space in the District of Columbia . AIDS and Behavior , 20 ( 12 ), 2933–2940. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Aral SO, O’Leary A, & Baker C (2020). Sexually transmitted infections and HIV in the southern United States: An overview . Sexually Transmitted Diseases , 33 ( 7 ), S1–S5. doi: 10.1097/01.olq.0000223249.04456.76 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arnold EA, Kegeles SM, Pollack LM, Neilands TB, Cornwell SM, Stewart WR, Benjamin M, Weeks J, Lockett G, Smith CD, & Operario D (2019). A randomized controlled trial to reduce HIV-related risk in African American men who have sex with men and women: The Bruthas project . Prevention Science , 20 ( 1 ), 115–125. 10.1007/s11121-018-0965-7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Azjen I (1991). The theory of planned behavior . Organizational Behavior and Human Decision Processes , 50 ( 2 ), 179–211. [ Google Scholar ]
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, … & Celum C. (2012). Antiretroviral prophylaxis for HIV prevention in heterosexual men and women . New England Journal of Medicine , 367 ( 5 ), 399–410. 10.1056/NEJMoa1108524 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bagchi AD (2020). A structural competency curriculum for primary care providers to address the opioid use disorder, HIV, and hepatitis C syndemic . Frontiers in Public Health , 8 , 210. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bailey J, Mann S, Wayal S, Hunter R, Free C, Abraham C, & Murray E (2015). Sexual health promotion for young people delivered via digital media: A scoping review . 10.3310/phr03130 (Public Health Research) [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bandura A (1991). Social cognitive theory of self-regulation . Organizational behavior and human decision processes , 50 ( 2 ), 248–287. [ Google Scholar ]
- Bandura A (2001). Social cognitive theory: An agentic perspective . Annual Review of Psychology , 52 ( 1 ), 1–26. 10.1146/annurev.psych.52.1.1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barry MC, Threats M, Blackburn NA, LeGrand S, Dong W, Pulley DV, Sallabank G, Harper GW, Hightow-Weidman LB, Bauermeister JA, & Muessig KE (2018). “Stay strong! Keep ya head up! Move on! It gets better!!!!”: Resilience processes in the healthMpowerment online intervention of young black gay, bisexual and other men who have sex with men . AIDS Care , 30 ( Suppl 5 ), S27–S38. 10.1080/09540121.2018.1510106 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bauermeister JA, Connochie D, Eaton L, Demers M, & Stephenson R (2017). Geospatial indicators of space and place: A review of multilevel studies of HIV prevention and care outcomes among young men who have sex with men in the United States . The Journal of Sex Research , 54 ( 4–5 ), 446–464. 10.1080/00224499.2016.1271862 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bauermeister JA, Golinkoff JM, Horvath KJ, Hightow-Weidman LB, Sullivan PS, & Stephenson R (2018). A multilevel tailored web app-based intervention for linking young men who have sex with men to quality care (get connected): Protocol for a randomized controlled trial . Journal of Medical Internet Research , 20 ( 8 ), 77. 10.2196/10444 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bauermeister JA, Tingler RC, Demers M, Connochie D, Gillard G, Shaver J, Chavanduka T, & Harper GW (2019). Acceptability and preliminary efficacy of an online HIV prevention intervention for single young men who have sex with men seeking partners online: The myDEx Project . AIDS and Behavior , 23 ( 11 ), 3064–3077. 10.1007/s10461-019-02426-7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bekker L-G, Beyrer C, & Quinn TC (2012). Behavioral and biomedical combination strategies for HIV prevention . Cold Spring Harbor Perspectives in Medicine , 2 ( 8 ), a007435. 10.1101/cshperspect.a007435 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Belenko S, Visher C, Pearson F, Swan H, Pich M, O’Connell D, Dembo R, Frisman L, Hamilton L, & Willett J (2017). Efficacy of structured organizational change intervention on HIV testing in correctional facilities . AIDS Education and Prevention , 29 ( 3 ), 241–255. [ PubMed ] [ Google Scholar ]
- Belgrave FZ, & Abrams JA (2016). Reducing disparities and achieving equity in African American women’s health . American Psychologist , 71 ( 8 ), 723–733. 10.1037/amp0000081 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Berkley-Patton J, Thompson CB, Moore E, Hawes S, Simon S, Goggin K, Martinez D, Berman M, & Booker A (2016). An HIV testing intervention in African American churches: Pilot study findings . Annals of Behavioral Medicine , 50 ( 3 ), 480–485. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Berkley-Patton JY, Thompson CB, Moore E, Hawes S, Berman M, Allsworth J, Williams E, Wainright C, Bradley-Ewing A, Bauer AG, Catley D, & Goggin K (2019). Feasibility and outcomes of an HIV testing intervention in African American churches . AIDS and Behavior , 23 ( 1 ), 76–90. 10.1007/s10461-018-2240-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bernstein J, Dorfman D, Lunstead J, Topp D, Mamata H, Jaffer S, & Bernstein E (2017). Reaching adolescents for prevention: The role of pediatric emergency department health promotion advocates . Pediatric Emergency Care , 33 ( 4 ), 223–229. [ PubMed ] [ Google Scholar ]
- Besoain F, Perez-Navarro A, Cayl JA, Avi CJ, & de Olalla P. G. a. (2015). Prevention of sexually transmitted infections using mobile devices and ubiquitous computing [Report] . International Journal of Health Geographics . 10.1186/s12942-015-0010-z [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blankenship KM, Reinhard E, Sherman SG, & El-Bassel N (2015). Structural interventions for HIV prevention among women who use drugs: A global perspective . JAIDS Journal of Acquired Immune Deficiency Syndromes , 69 , S140–S145. 10.1097/qai.0000000000000638 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bond KT, & Gunn AJ (2016). Perceived advantages and disadvantages of using pre-exposure prophylaxis (PrEP) among sexually active Black women: An exploratory study . Journal of Black Sexuality and Relationships , 3 ( 1 ), 1–24. 10.1353/bsr.2016.0019 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bond KT, & Ramos SR (2019). Utilization of an animated electronic health video to increase knowledge of post- and pre-exposure prophylaxis for HIV among African American women: Nationwide cross-sectional survey . JMIR Formative Research , 3 ( 2 ), e9995. 10.2196/formative.9995 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Boni RB, Lentini N, Santelli C, Barbosa A, Cruz M, Bingham T, Cota V, Correa RG, Veloso VG, & Grinsztejn B (2018). Self-testing, communication and information technology to promote HIV diagnosis among young gay and other men who have sex with men (MSM) in Brazil . Journal of the International AIDS Society , 21 ( Suppl 5 ), e25116. 10.1002/jia2.25116 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brawner BM (2014). A multilevel understanding of HIV/AIDS disease burden among African American women . Journal of Obstetric, Gynecologic, and Neonatal Nursing , 43 ( 5 ), 633–643. 10.1111/1552-6909.12481 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brawner BM, Abboud S, Reason J, Wingood G, & Jemmott LS (2019). The development of an innovative, theory-driven, psychoeducational HIV/STI prevention intervention for heterosexually active black adolescents with mental illnesses . Vulnerable Children and Youth Studies , 14 ( 2 ), 151–165. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brawner BM, Guthrie B, Stevens R, Taylor L, Eberhart M, & Schensul JJ (2017). Place still matters: Racial/ethnic and geographic disparities in HIV transmission and disease burden . Journal of Urban Health , 94 , 716–729. 10.1007/s11524-017-0198-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brawner BM, Reason JL, Goodman BA, Schensul JJ, & Guthrie B (2015). Multilevel drivers of human immunodeficiency virus/acquired immune deficiency syndrome among Black Philadelphians: Exploration using community ethnography and geographic information systems . Nursing Research , 64 ( 2 ), 100–110. 10.1097/NNR.0000000000000076 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brewer R, Daunis C, Ebaady S, Wilton L, Chrestman S, Mukherjee S, Moore M, Corrigan R, & Schneider J (2019). Implementation of a socio-structural demonstration project to improve HIV outcomes among young Black men in the deep south . Journal of Racial and Ethnic Health Disparities , 6 ( 4 ), 775–789. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bunting SR, Saqueton R, & Batteson TJ (2020). Using a student-led, community-specific training module to increase PrEP uptake amongst at-risk populations: Results from an exploratory pilot implementation . AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV , 32 ( 5 ), 546–550. [ PubMed ] [ Google Scholar ]
- Cahill S, Taylor SW, Elsesser SA, Mena L, Hickson D, & Mayer KH (2017). Stigma, medical mistrust, and perceived racism may affect PrEP awareness and uptake in black compared to white gay and bisexual men in Jackson, Mississippi and Boston, Massachusetts . AIDS Care , 29 ( 11 ), 1351–1358. 10.1080/09540121.2017.1300633 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Carrico AW, Jain J, Discepola MV, Olem D, Andrews R, Woods WJ, Neilands TB, Shoptaw S, Gómez W, Dilworth SE, & Moskowitz JT (2016). A community-engaged randomized controlled trial of an integrative intervention with HIV-positive, methamphetamine-using men who have sex with men . BMC Public Health , 16 , 673. 10.1186/s12889-016-3325-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Catania JA, Kegeles SM, & Coates TJ (1990). Towards an understanding of risk behavior: An AIDS risk reduction model (ARRM) . Health Education Quarterly , 17 ( 1 ), 53–72. 10.1177/109019819001700107 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Centers for Disease Control and Prevention. (2020). Compendium of evidence-based interventions and best practices for HIV prevention . Retrieved from: https://www.cdc.gov/hiv/research/interventionresearch/compendium/index.html
- Chandler R, Hull S, Ross H, Guillaume D, Paul S, Dera N, & Hernandez N (2020). The pre-exposure prophylaxis (PrEP) consciousness of black college women and the perceived hesitancy of public health institutions to curtail HIV in black women . BMC Public Health , 20 ( 1 ), 1172. 10.1186/s12889-020-09248-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cho H, Powell D, Pichon A, Kuhns LM, Garofalo R, & Schnall R (2019). Eye-tracking retrospective think-aloud as a novel approach for a usability evaluation . International Journal of Medical Informatics , 129 , 366–373. 10.1016/j.ijmedinf.2019.07.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cho H, Powell D, Pichon A, Thai J, Bruce J, Kuhns LM, Garofalo R, & Schnall R (2018). A mobile health intervention for HIV prevention among racially and ethnically diverse young men: Usability evaluation . JMIR Mhealth Uhealth , 6 ( 9 ), e11450. 10.2196/11450 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Coates TJ, Richter L, & Caceres C (2008). Behavioural strategies to reduce HIV transmission: How to make them work better . Lancet , 372 ( 9639 ), 669–684. 10.1016/S0140-6736(08)60886-7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Coffin PO, Rowe C, & Santos GM (2015). Novel interventions to prevent HIV and HCV among persons who inject drugs . Current HIV/AIDS Reports , 12 ( 1 ), 145–163. 10.1007/s11904-014-0248-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JHS, Godbole SV, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Cottle L, Zhang XC, Makhema J, … & Fleming TR (2016). Antiretroviral therapy for the prevention of HIV-1 transmission . New England Journal of Medicine , 375 ( 9 ), 830–839. 10.1056/NEJMoa1600693 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Colarossi LG, Hazel DS, Collier KL, Desouza S, & Pappas L (2016). Research with Latina and Black women for an HIV prevention campaign . Social Marketing Quarterly , 22 ( 3 ), 236–252. [ Google Scholar ]
- Collins LM, Kugler KC, & Gwadz MV (2016). Optimization of multicomponent behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS . AIDS and Behavior , 20 ( 1 ), 197–214. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Conserve DF, Jennings L, Aguiar C, Shin G, Handler L, & Maman S (2016). Systematic review of mobile health behavioural interventions to improve uptake of HIV testing for vulnerable and key populations . Journal of Telemedicine and Telecare , 23 ( 2 ), 347–359. 10.1177/1357633X16639186 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cordova D, Alers-Rojas F, Lua FM, Bauermeister J, Nurenberg R, Ovadje L, Fessler K, Delva J, Salas-Wright CP, & Council YL (2018). The usability and acceptability of an adolescent mHealth HIV/STI and drug abuse preventive intervention in primary care . Behavioral Medicine , 44 ( 1 ), 36–47. 10.1080/08964289.2016.1189396 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Côté J, Rouleau G, Ramirez-Garcia P, & Bourbonnais A (2015). Virtual nursing intervention adjunctive to conventional care: The experience of persons living with HIV . JMIR Research Protocols , 4 ( 4 ), e124. 10.2196/resprot.4158 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Crosby RA, Mena L, & Smith RV (2018). Promoting positive condom use experiences among young black MSM: A randomized controlled trial of a brief, clinic-based intervention . Health Education Research , 33 ( 3 ), 197–204. 10.1093/her/cyy010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Crosby R, DiClemente RJ, Charnigo R, Snow G, & Troutman A (2009). A brief, clinic-based, safer sex intervention for heterosexual African American men newly diagnosed with an STD: A randomized controlled trial . American Journal of Public Health , 99 ( Suppl 1 ), S96–S103. 10.2105/AJPH.2007.123893 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Danielson CK, McCauley JL, Gros KS, Jones AM, Barr SC, Borkman AL, Bryant BG, & Ruggiero KJ (2014). SiHLEWeb.com: Development and usability testing of an evidence-based HIV prevention website for female African-American adolescents . Health Informatics Journal , 22 ( 2 ), 194–208. 10.1177/1460458214544048 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- De Boni RB, Lentini N, Santelli AC, Barbosa A Jr., Cruz M, Bingham T, Cota V, Correa RG, Veloso VG, & Grinsztejn B (2018). Self-testing, communication and information technology to promote HIV diagnosis among young gay and other men who have sex with men (MSM) in Brazil . Journal of the International AIDS Society , 21 ( Suppl 5 ), e25116. 10.1002/jia2.25116 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Derose KP, Griffin BA, Kanouse DE, Bogart LM, Williams MV, Haas AC, Flórez KR, Collins DO, Hawes-Dawson J, Mata MA, Oden CW, & Stucky BD (2016). Effects of a pilot church-based intervention to reduce HIV stigma and promote HIV testing among African Americans and Latinos . AIDS and Behavior , 20 ( 8 ), 1692–1705. 10.1007/s10461-015-1280-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Deutsch MB, Glidden DV, Sevelius J, Keatley J, McMahan V, Guanira J, Kallas EG, Chariyalertsak S, & Grant RM (2015). HIV pre-exposure prophylaxis in transgender women: A subgroup analysis of the iPrEx trial . The Lancet HIV , 2 ( 12 ), e512–e519. 10.1016/S2352-3018(15)00206-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- DiClemente RJ, Wingood GM, Rose ES, Sales JM, Lang DL, Caliendo AM, Hardin JW, & Crosby RA (2009). Efficacy of sexually transmitted disease/human immunodeficiency virus sexual risk-reduction intervention for African American adolescent females seeking sexual health services: A randomized controlled trial . Archives of Pediatrics & Adolescent Medicine , 163 ( 12 ), 1112–1121. 10.1001/archpediatrics.2009.205 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dietrich JJ, Lazarus E, Andrasik M, Hornschuh S, Otwombe K, Morgan C, & Isaacs AJ (2018). Mobile phone questionnaires for sexual risk data collection among young women in Soweto, South Africa . AIDS and Behavior , 7 , 2312. 10.1007/s10461-018-2080-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dillingham R, Ingersoll K, Flickinger TE, Waldman AL, Grabowski M, Laurence C, Wispelwey E, Reynolds G, Conaway M, & Cohn WF (2018). PositiveLinks: A mobile health intervention for retention in HIV care and clinical outcomes with 12-month follow-up . AIDS Patient Care and STDs , 32 ( 6 ), 241–250. 10.1089/apc.2017.0303 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- DiNenno EA, Prejean J, Irwin K, Delaney KP, Bowles K, Martin T, Tailor A, Dumitru G, Mullins MM, Hutchinson AB, & Lansky A (2017). Recommendations for HIV screening of gay, bisexual, and other men who have sex with men—United States, 2017 . MMWR. Morbidity and Mortality Weekly Report , 66 ( 31 ), 830–832. 10.15585/mmwr.mm6631a3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Do TTT, Le MD, Van Nguyen T, Tran BX, Le HT, Nguyen HD, Nguyen LH, Nguyen CT, Tran TD, Latkin CA, Ho RCM, & Zhang MWB (2018). Receptiveness and preferences of health-related smartphone applications among Vietnamese youth and young adults . BMC Public Health , 18 ( 1 ), 764. 10.1186/s12889-018-5641-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Donenberg G, Emerson E, & Kendall AD (2018). HIV-risk reduction intervention for juvenile offenders on probation: The PHAT Life group randomized controlled trial . Health Psychology , 37 ( 4 ), 364–374. 10.1037/hea0000582 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Duarte G, Vanegas J, Bravo G, Rada G, & Pantoja T (2019). Effectiveness of digital interventions based on mobile phones for the prevention of sexually transmitted infections: A systematic review protocol . Medwave , 19 ( 2 ), e7605. 10.5867/medwave.2019.02.7605 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Duncan DT, Hickson DA, Goedel WC, Callander D, Brooks B, Chen YT, Hanson H, Eavou R, Khanna AS, Chaix B, Regan SD, Wheeler DP, Mayer KH, Safren SA, Melvin SC, Draper C, Magee-Jackson V, Brewer R, Schneider JA, on behalf of the, N., & Network Cohort Study, T. (2019). The social context of HIV prevention and care among black men who have sex with men in three U.S. cities: The neighborhoods and networks (N2) cohort study . International Journal of Environmental Research and Public Health , 16 ( 11 ), e1922. 10.3390/ijerph16111922 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eberhart MG, Yehia BR, Hillier A, Voytek CD, Fiore DJ, Blank M, Frank I, Metzger DS, & Brady KA (2015). Individual and community factors associated with geographic clusters of poor HIV care retention and poor viral suppression . Journal of Acquired Immune Deficiency Syndromes , 69 , S37–S43. 10.1097/QAI.0000000000000587 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eke AN, Johnson WD, O’Leary A, Rebchook GM, Huebner DM, Peterson JL, & Kegeles SM (2019). Effect of a community-level HIV prevention intervention on psychosocial determinants of HIV risk behaviors among young Black men who have sex with men (YBMSM) . AIDS and Behavior , 23 ( 9 ), 2361–2374. [ PubMed ] [ Google Scholar ]
- El-Bassel N, & Strathdee SA (2015). Women who use or inject drugs: An action agenda for women-specific, multilevel, and combination HIV prevention and research . Journal of Acquired Immune Deficiency Syndromes , 69 ( Suppl 2 ), S182–S190. 10.1097/QAI.0000000000000628 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- El-Sadr WM, Donnell D, Beauchamp G, Hall HI, Torian LV, Zingman B, Lum G, Kharfen M, Elion R, Leider J, Gordin FM, Elharrar V, Burns D, Zerbe A, Gamble T, & Branson B (2017). Financial incentives for linkage to care and viral suppression among HIV-positive patients: A randomized clinical trial (HPTN 065) . JAMA Internal Medicine , 177 ( 8 ), 1083–1092. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Enah C, Lambert C, Gakumo CA, Daniel M, & Wehby PS (2019). Working with potential users to develop an HIV/STI prevention video game for rural adolescents . Clinical HIV and AIDS Journal , 2 ( 1 ), 108. [ Google Scholar ]
- Enah C, Moneyham L, Vance DE, & Childs G (2013). Digital gaming for HIV prevention with young adolescents . The Journal of the Association of Nurses in AIDS Care: JANAC , 24 ( 1 ), 71–80. 10.1016/j.jana.2012.03.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Enah C, Piper K, & Moneyham L (2015). Qualitative evaluation of the relevance and acceptability of a web-based HIV prevention game for rural adolescents . Journal of pediatric nursing , 30 ( 2 ), 321–328. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Erguera XA, Johnson MO, Neilands TB, Ruel T, Berrean B, Thomas S, & Saberi P (2019). WYZ: A pilot study protocol for designing and developing a mobile health application for engagement in HIV care and medication adherence in youth and young adults living with HIV . BMJ Open , 9 ( 5 ), e030473. 10.1136/bmjopen-2019-030473 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fan X, She R, Liu C, Zhong H, Lau JTF, Hao C, Li J, Hao Y, Li L, & Gu J (2020). Evaluation of smartphone APP-based case-management services among antiretroviral treatment-naive HIV-positive men who have sex with men: A randomized controlled trial protocol . BMC Public Health , 20 ( 1 ), 1–13. 10.1186/s12889-020-8171-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fauci AS, Redfield RR, Sigounas G, Weahkee MD, & Giroir BP (2019). Ending the HIV epidemic: A plan for the United States . Journal of the American Medical Association , 321 ( 9 ), 844–845. [ PubMed ] [ Google Scholar ]
- Fishbein M, & Ajzen I (1975). Belief, attitude, intention and behavior: An introduction to theory and research . Addison-Wesley. [ Google Scholar ]
- Flores DD, Rosario AA, Bond KT, Villarruel AM, & Bauermeister JA (2020). Parents ASSIST (Advancing Supportive and Sexuality-Inclusive Sex Talks): Iterative development of a sex communication video series for parents of gay, bisexual, and queer male adolescents . Journal of Family Nursing , 26 ( 2 ), 90–101. 10.1177/1074840719897905 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fogel CI, Crandell JL, Neevel AM, Parker SD, Carry M, White BL, Fasula AM, Herbst JH, & Gelaude DJ (2015). Efficacy of an adapted HIV and sexually transmitted infection prevention intervention for incarcerated women: A randomized controlled trial . American Journal of Public Health , 105 ( 4 ), 802–809. 10.2105/AJPH.2014.302105 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Frew PM, Parker K, Vo L, Haley D, O’Leary A, Diallo DD, Golin CE, Kuo I, Soto-Torres L, Wang J, Adimora AA, Randall LA, del Rio C, Hodder S, & the, H. I. V. P. T. N. S. T (2016). Socioecological factors influencing women’s HIV risk in the United States: Qualitative findings from the women’s HIV SeroIncidence study (HPTN 064) . BMC Public Health , 16 ( 1 ), 803. 10.1186/s12889-016-3364-7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Frye V, Paige MQ, Gordon S, Matthews D, Musgrave G, Greene E, Kornegay M, Farhat D, Smith PH, Usher D, Phelan JC, Koblin BA, & Taylor-Akutagawa V (2019). Impact of a community-level intervention on HIV stigma, homophobia and HIV testing in New York City: Results from project CHHANGE . Stigma and Health , 4 ( 1 ), 72–81. 10.1037/sah0000109 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gabarron E, & Wynn R (2016). Use of social media for sexual health promotion: A scoping review . Global Health Action , 9 ( 1 ), 32193. 10.3402/gha.v9.32193 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gamble T, Branson B, Donnell D, Hall HI, King G, Cutler B, Hader S, Burns D, Leider J, Wood AF, Volpp KG, Buchacz K, & El-Sadr WM (2017). Design of the HPTN 065 (TLC-Plus) study: A study to evaluate the feasibility of an enhanced test, link-to-care, plus treat approach for HIV prevention in the United States . Clinical Trials , 14 ( 4 ), 322–332. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gardner EM, McLees MP, Steiner JF, Del Rio C, & Burman WJ (2011). The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection . Clinical Infectious Diseases , 52 ( 6 ), 793–800. 10.1093/cid/ciq243 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garett R, Smith J, & Young SD (2016). A review of social media technologies across the global HIV care continuum . Current Opinion in Psychology , 9 , 56–66. 10.1016/j.copsyc.2015.10.024 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Golub SA, Fikslin RA, Starbuck L, & Klein A (2019). High rates of PrEP eligibility but low rates of PrEP access among a national sample of transmasculine individuals . Journal of Acquired Immune Deficiency Syndromes , 82 ( 1 ), e1–e7. https://journals.lww.com/jaids/Fulltext/2019/09010/High_Rates_of_PrEP_Eligibility_but_Low_Rates_of.6.aspx [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T,Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker L-G, Mayer KH, Kallás EG, Amico KR, … & Glidden DV (2010). Preexposure chemoprophylaxis for HIV prevention in men who have sex with men . New England Journal of Medicine , 363 ( 27 ), 2587–2599. 10.1056/NEJMoa1011205 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gregg E, Linn C, Nace E, Gelberg L, Cowan B, & Fulcher JA (2020). Implementation of HIV preexposure prophylaxis in a homeless primary care setting at the Veterans Affairs . Journal of Primary Care & Community Health , 11 , 2510132720908370. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hadley W, Brown LK, Barker D, Warren J, Weddington P, Fortune T, & Juzang I (2016). Work It Out Together: Preliminary efficacy of a parent and adolescent DVD and workbook intervention on adolescent sexual and substance use attitudes and parenting behaviors . AIDS and Behavior , 20 ( 9 ), 1961–1972. 10.1007/s10461-016-1418-6 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Havens JP, Scarsi KK, Sayles H, Klepser DG, Swindells S, & Bares SH (2019). Acceptability and feasibility of a pharmacist-led human immunodeficiency virus pre-exposure prophylaxis program in the Midwestern United States . Open Forum Infectious Diseases , 6 ( 10 ), ofz365. 10.1093/ofid/ofz365 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Henny KD, Wilkes AL, McDonald CM, Denson DJ, & Neumann MS (2018a). A Rapid Review of eHealth Interventions Addressing the Continuum of HIV Care (2007–2017) . AIDS and Behavior , 22 ( 1 ), 43–63. 10.1007/s10461-017-1923-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hess KL, Hu X, Lansky A, Mermin J, & Hall HI (2017). Lifetime risk of a diagnosis of HIV infection in the United States . Annals of Epidemiology , 27 ( 4 ), 238–243. 10.1016/j.annepidem.2017.02.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hightow-Weidman LB, & Bauermeister JA (2020). Engagement in mHealth behavioral interventions for HIV prevention and care: Making sense of the metrics . MHealth , 6 , 7. 10.21037/mhealth.2019.10.01 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hightow-Weidman LB, Muessig KE, Bauermeister JA, LeGrand S, & Fiellin LE (2017). The future of digital games for HIV prevention and care . Current Opinion in HIV and AIDS , 12 ( 5 ), 501–507. 10.1097/COH.0000000000000399 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hightow-Weidman L, Muessig K, Claude K, Roberts J, Zlotorzynska M, & Sanchez T (2020). Maximizing digital interventions for youth in the midst of Covid-19: Lessons from the adolescent trials network for HIV interventions . AIDS and Behavior , 24 ( 8 ), 2239–2243. 10.1007/s10461-020-02870-w [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hightow-Weidman L, Muessig K, Knudtson K, Srivatsa M, Lawrence E, LeGrand S, Hotten A, & Hosek S (2018). A gamified smartphone app to support engagement in care and medication adherence for HIV-positive young men who have sex with men (AllyQuest): Development and pilot study . JMIR Public Health and Surveillance , 4 ( 2 ), e34. 10.2196/publichealth.8923 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hill MJ, Holt M, Hanscom B, Wang Z, Cardenas-Turanzas M,& Latkin C (2018). Gender and race as correlates of high risk sex behaviors among injection drug users at risk for HIV enrolled in the HPTN 037 study . Drug and Alcohol Dependence , 183 , 267–274. 10.1016/j.drugalcdep.2017.11.018 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hiransuthikul A, Janamnuaysook R, Himmad K, Kerr SJ, Thammajaruk N, Pankam T, Phanjaroen K, Mills S, Vannakit R, Phanuphak P, & Phanuphak N (2019). Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: The iFACT study . Journal of the International AIDS Society , 22 ( 7 ), e25338. 10.1002/jia2.25338 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- HIV Prevention Trials Network. (2020). A phase 3 double blind safety and efficacy study of long-acting injectable cabotegravir compared to daily oral TDF/FTC for pre-exposure prophylaxis in HIV-uninfected women . Retrieved from https://www.hptn.org/research/studies/hptn084
- Hojilla JC, Marcus JL, Silverberg MJ, Hare CB, Herbers R, Hurley L, Satre DD, & Volk JE (2020). Early adopters of event-driven human immunodeficiency virus pre-exposure prophylaxis in a large healthcare system in San Francisco . Clinical Infectious Diseases , 71 ( 10 ), 2710–2712. 10.1093/cid/ciaa474 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, & Carrico A (2018). HIV pre-exposure prophylaxis (PrEP) uptake and retention among men who have sex with men in a community-based sexual health clinic . AIDS and Behavior , 22 ( 4 ), 1096–1099. 10.1007/s10461-017-2009-x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Holloway IW, Winder TJ, Lea CH III, Tan D, Boyd D, & Novak D (2017). Technology use and preferences for mobile phone-based HIV prevention and treatment among Black young men who have sex with men: Exploratory research . JMIR mHealth and uHealth , 5 ( 4 ), e46. 10.2196/mhealth.6436 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Holtgrave DR, Maulsby C, Kim JJ, Cassidy-Stewart H, & Hauck H (2016). Using resource allocation modeling to inform HIV prevention priority setting for Baltimore-Towson, Maryland . Progress in Community Health Partnerships: Research, Education, and Action , 10 ( 1 ), 133–139. [ PubMed ] [ Google Scholar ]
- Horvath KJ, Walker T, Mireles L, Bauermeister JA, Hightow-Weidman L, & Stephenson R (2020). A systematic review of technology-assisted HIV testing interventions . Current HIV/AIDS Reports , 17 ( 4 ), 269–280. 10.1007/s11904-020-00506-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hosek SG, Green KR, Siberry G, Lally M, Balthazar C, Serrano PA, Kapogiannis B, & The Adolescent Medicine Trials Network for, H. I. V. A. I. (2013). Integrating behavioral HIV interventions into biomedical prevention trials with youth: Lessons from Chicago’s Project PrEPare . Journal of HIV/AIDS & Social Services , 12 ( 3–4 ), 333–348. 10.1080/15381501.2013.773575 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hosek SG, Rudy B, Landovitz R, Kapogiannis B, Siberry G, Rutledge B, Liu N, Brothers J, Mulligan K, Zimet G, Lally M, Mayer KH, Anderson P, Kiser J, Rooney JF, Wilson CM, & Adolescent Trials Network for, H. I. (2017). An HIV preexposure prophylaxis demonstration project and safety study for young MSM . Journal of Acquired Immune Deficiency Syndromes , 74 ( 1 ), 21–29. 10.1097/QAI.0000000000001179 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hosek SG, Siberry G, Bell M, Lally M, Kapogiannis B, Green K, Fernandez MI, Rutledge B, Martinez J, Garofalo R, Wilson CM, & Adolescent Trials Network for, H. I. (2013). The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men . Journal of Acquired Immune Deficiency Syndromes , 62 ( 4 ), 447–456. 10.1097/QAI.0b013e3182801081 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoth AB, Shafer C, Dillon DB, Mayer R, Walton G, & Ohl ME (2019). Iowa TelePrEP: A public-health-partnered telehealth model for human immunodeficiency virus preexposure prophylaxis delivery in a rural state . Sexually Transmitted Diseases , 46 ( 8 ), 507–512. [ PubMed ] [ Google Scholar ]
- Houck CD, Hadley W, Barker D, Brown LK, Hancock E, & Almy B (2016). An emotion regulation intervention to reduce risk behaviors among at-risk early adolescents . Prevention Science , 17 ( 1 ), 71–82. 10.1007/s11121-015-0597-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jadwin-Cakmak L, Bauermeister JA, Cutler JM, Loveluck J, Kazaleh Sirdenis T, Fessler KB, Popoff EE, Benton A, Pomerantz NF, Gotts Atkins SL, Springer T, & Harper GW (2020). The Health Access Initiative: A training and technical assistance program to improve health care for sexual and gender minority youth . Journal of Adolescent Health , 67 ( 1 ), 115–122. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jaiswal J, Griffin M, Singer SN, Greene RE, Acosta ILZ, Kaudeyr SK, Kapadia F, & Halkitis PN (2018). Structural barriers to pre-exposure prophylaxis use among young sexual minority men: The P18 cohort study . Current HIV Research , 16 ( 3 ), 237–249. 10.2174/1570162X16666180730144455 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jemmott JB III, Jemmott LS, & Fong GT (1992). Reductions in HIV risk-associated sexual behaviors among black male adolescents: Effects of an AIDS prevention intervention . American Journal of Public Health , 82 ( 3 ), 372–377. 10.2105/AJPH.82.3.372 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jemmott JB III, Jemmott LS, & Fong GT (1998). Abstinence and safer sex HIV risk-reduction interventions for African American adolescents: A randomized controlled trial . JAMA , 279 ( 19 ), 1529–1536. 10.1001/jama.279.19.1529 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jemmott JB III, Jemmott LS, & Fong GT (2010). Efficacy of a theory-based abstinence-only intervention over 24 months: A randomized controlled trial with young adolescents . Archives of Pediatrics & Adolescent Medicine , 164 ( 2 ), 152–159. 10.1001/archpediatrics.2009.267 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jemmott JB III, Jemmott LS, Fong GT, & McCaffree K (1999). Reducing HIV risk-associated sexual behavior among African American adolescents: Testing the generality of intervention effects . American Journal of Community Psychology , 27 ( 2 ), 161–187. 10.1007/BF02503158 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jemmott LS, Jemmott JB III, Chittamuru D, & Icard LD (2019). Effects of a sexual HIV risk reduction intervention for African American mothers and their adolescent sons: A randomized controlled trial . Journal of Adolescent Health , 65 ( 5 ), 643–650. 10.1016/j.jadohealth.2019.05.017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jemmott LS, Jemmott JB III, Icard LD, & Hsu J (2020). Effects of church-based parent–child abstinence-only interventions on adolescents’ sexual behaviors . Journal of Adolescent Health , 66 ( 1 ), 107–114. 10.1016/j.jadohealth.2019.07.021 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jemmott LS, Jemmott JB III, & O’Leary A (2007). Effects on sexual risk behavior and STD rate of brief HIV/STD prevention interventions for African American women in primary care settings . American Journal of Public Health , 97 ( 6 ), 1034–1040. 10.2105/AJPH.2003.020271 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jongbloed K, Parmar S, Kop M, Spittal PM, & Lester RT (2015). Recent evidence for emerging digital technologies to support global HIV engagement in care . Current HIV/AIDS Reports , 12 ( 4 ), 451–461. 10.1007/s11904-015-0291-7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kamitani E, Mizuno Y, Wichser M, Adegbite AH, DeLuca JB, & Higa DH (2019). Mapping the study characteristics and topics of HIV pre-exposure prophylaxis research literature: A scoping review . AIDS Education and Prevention , 31 ( 6 ), 505–522. 10.1521/aeap.2019.31.6.505 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Katz DA, Dombrowski JC, Barry M, Spellman D, Bell TR, & Golden MR (2019). STD partner services to monitor and promote HIV pre-exposure prophylaxis use among men who have sex with men . Journal of Acquired Immune Deficiency Syndromes , 80 ( 5 ), 533–541. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kay ES, Batey DS, & Mugavero MJ (2016). The HIV treatment cascade and care continuum: Updates, goals, and recommendations for the future . AIDS Research and Therapy , 13 , 35. 10.1186/s12981-016-0120-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kemp CG, & Velloza J (2018). Implementation of eHealth interventions across the HIV care cascade: A review of recent research . Current HIV/AIDS Reports , 15 ( 6 ), 403–413. 10.1007/s11904-018-0415-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kerr JC, Valois RF, Diclemente RJ, Carey MP, Stanton B, Romer D, Fletcher F, Farber N, Brown LK, Vanable PA, Salazar LF, Juzang I, & Fortune T (2015). The effects of a mass media HIV-risk reduction strategy on HIV-related stigma and knowledge among African American adolescents . AIDS Patient Care and STDs , 29 ( 3 ), 150–156. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kibengo FM, Ruzagira E, Katende D, Bwanika AN, Bahemuka U, Haberer JE, Bangsberg DR, Barin B, Rooney JF, Mark D, Chetty P, Fast P, Kamali A, & Priddy FH (2013). Safety, adherence and acceptability of intermittent tenofovir/emtricitabine as HIV pre-exposure prophylaxis (PrEP) among HIV-uninfected Ugandan volunteers living in HIV-serodiscordant relationships: A randomized, clinical trial . PLoS One , 8 ( 9 ), e74314. 10.1371/journal.pone.0074314 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kuhns LM, Hotton AL, Perloff J, Paul J, Parker C, Muldoon AL, Johnson AK, & Garofalo R (2019). Evaluation of translife care: An intervention to address social determinants of engagement in HIV care among transgender women of color . AIDS and Behavior . 10.1007/s10461-019-02548-y [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kuo CC, Rosen RK, Zlotnick C, Wechsberg WM, Peabody M, & Johnson JE (2019). Sexual health prevention for incarcerated women: Eroticising safe sex during re-entry to the community . Journal of Family Planning & Reproductive Health Care , 45 ( 1 ), 17–22. 10.1136/bmjsrh-2017-200024 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kurth AE, Celum C, Baeten JM, Vermund SH, & Wasserheit JN (2011). Combination HIV prevention: Significance, challenges, and opportunities . Current HIV/AIDS Reports , 8 ( 1 ), 62–72. 10.1007/s11904-010-0063-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Landovitz RJ., Donnell D, Clement M, Hanscom H, Cottle L, Coelho L, et al. (2020). HPTN083 interim results: Pre-exposure prophylaxis (PrEP) containing long-acting injectable cabotegravir (CAB-LA) is safe and highly effective for cisgender men and transgender women who have sex with men (MSM,TGW) . IAS Conference, San Francisco . 2020. [ Google Scholar ]
- Lanier Y, & Sutton MY (2013). Reframing the context of preventive health care services and prevention of HIV and other sexually transmitted infections for young men: New opportunities to reduce racial/ethnic sexual health disparities . American Journal of Public Health , 103 ( 2 ), 262–269. 10.2105/AJPH.2012.300921 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lauby J, Zhu L, Milnamow M, Batson H, Bond L, Curran-Groome W, & Carson L (2017). Get real: Evaluation of a community level HIV prevention intervention for young MSM who engage in episodic substance use . AIDS Education and Prevention , 29 ( 3 ), 191–204. [ PubMed ] [ Google Scholar ]
- LeGrand S, Knudtson K, Benkeser D, Muessig K, McGee A, Sullivan PS, & Hightow-Weidman L (2018). Testing the efficacy of a social networking gamification app to improve pre-exposure prophylaxis adherence (P3: Prepared, Protected, emPowered): Protocol for a randomized controlled trial . JMIR Research Protocols , 7 ( 12 ), e10448. 10.2196/10448 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Levy ME, Watson CC, Glick SN, Kuo I, Wilton L, Brewer RA, Fields SD, Criss V, & Magnus M (2016). Receipt of HIV prevention interventions is more common in community-based clinics than in primary care or acute care settings for Black men who have sex with men in the District of Columbia . AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV , 28 ( 5 ), 660–664. 10.1080/09540121.2015.1120266 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lewis CF, Lekas HM, Rivera A, Williams SZ, Crawford ND, Pérez-Figueroa RE, Joseph AM, & Amesty S (2020). Pharmacy PEP access intervention among persons who use drugs in New York City: iPEPcare Study—Rethinking biomedical HIV prevention strategies . AIDS and Behavior , 24 ( 7 ), 2101–2111. [ PubMed ] [ Google Scholar ]
- Lewis CF, Rivera AV, Crawford ND, DeCuir J, & Amesty S (2015). Pharmacy-randomized intervention delivering HIV prevention services during the syringe sale to people who inject drugs in New York City . Drug and Alcohol Dependence , 153 , 72–77. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Liang D, Han H, Du J, Zhao M, & Hser Y-I (2018). A pilot study of a smartphone application supporting recovery from drug addiction . Journal of Substance Abuse Treatment , 88 , 51–58. 10.1016/j.jsat.2018.02.006 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liran O, Dasher R, & Kaeochinda K (2019). Using virtual reality to improve antiretroviral therapy adherence in the treatment of HIV: Open-label repeated measure study . Interactive Journal of Medical Research , 8 ( 2 ), e13698. 10.2196/13698 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu AY, Vittinghoff E, von Felten P, Rivet Amico K, Anderson PL, Lester R, Andrew E, Estes I, Serrano P, Brothers J, Buchbinder S, Hosek S, & Fuchs JD (2019). Randomized controlled trial of a mobile health intervention to promote retention and adherence to preexposure prophylaxis among young people at risk for human immunodeficiency virus: The EPIC study . Clinical Infectious Diseases , 68 ( 12 ), 2010–2017. 10.1093/cid/ciy810 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lopez MI, Cocohoba J, Cohen SE, Trainor N, Levy MM, & Dong BJ (2020). Implementation of pre-exposure prophylaxis at a community pharmacy through a collaborative practice agreement with San Francisco Department of Public Health . Journal of the American Pharmacists Association: JAPHA , 60 ( 1 ), 138–144. 10.1016/j.japh.2019.06.021 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Los Angeles County Department of Public Health. (2019). What’s new in HIV PrEP? 2-1-1 dosing and FDA advisory panel recommendation new drug for PrEP . Retrieved February 9, 2021 from. http://rx.ph.lacounty.gov/RxHIV211PrEP0919 .
- MacLachlan JH, & Cowie BC (2015). Hepatitis B virus epidemiology . Cold Spring Harbor Perspectives in Medicine , 5 ( 5 ), a021410. 10.1101/cshperspect.a021410 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maloney KM, Bratcher A, Wilkerson R, & Sullivan PS (2020). Electronic and other new media technology interventions for HIV care and prevention: A systematic review . Journal of the International AIDS Society Electronic Resource , 23 ( 1 ), e25439. 10.1002/jia2.25439 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marcus JL, Hurley LB, Krakower DS, Alexeeff S, Silverberg MJ, & Volk JE (2019). Use of electronic health record data and machine learning to identify candidates for HIV pre-exposure prophylaxis: A modelling study . The Lancet HIV , 6 ( 10 ), e688–e695. 10.1016/S2352-3018(19)30137-7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Mâsse B, Grossman C, Rooney J, … & Chirenje ZM (2015). Tenofovir-based preexposure prophylaxis for HIV infection among African Women . New England Journal of Medicine , 372 ( 6 ), 509–518. 10.1056/NEJMoa1402269 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Chaipung B, Worrajittanon D, Leethochawalit M, Chiamwongpaet S, Kittimunkong S, Gvetadze RJ, McNicholl JM, Paxton LA, Curlin ME, Holtz TH, Samandari T, & Choopanya K (2017). Factors associated with the uptake of and adherence to HIV pre-exposure prophylaxis in people who have injected drugs: An observational, open-label extension of the Bangkok Tenofovir Study . The Lancet HIV , 4 ( 2 ), e59–e66. 10.1016/S2352-3018(16)30207-7 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mayer KH, Grinsztejn B, & El-Sadr WM (2016). Transgender people and HIV prevention: What we know and what we need to know, a call to action . Journal of Acquired Immune Deficiency Syndromes , 72 , S207–S209. 10.1097/QAI.0000000000001086 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mayer KH, Molina JM, Thompson MA, Anderson PL, Mounzer KC, De Wet JJ, DeJesus E, Jessen H, Grant RM, Ruane PJ, Wong P, Ebrahimi R, Zhong L, Mathias A, Callebaut C, Collins SE, Das M, McCallister S, Brainard DM, Brinson C, …& Hare CB (2020). Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): Primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial . Lancet , 396 ( 10246 ), 239–254. 10.1016/S0140-6736(20)31065-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mayo-Wilson LJ, Glass NE, Ssewamala FM, Linnemayr S, Coleman J, Timbo F, Johnson MW, Davoust M, Labrique A, Yenokyan G, Dodge B, & Latkin C (2019). Microenterprise intervention to reduce sexual risk behaviors and increase employment and HIV preventive practices in economically-vulnerable African-American young adults (EMERGE): Protocol for a feasibility randomized clinical trial . Trials Electronic Resource , 20 ( 1 ), 439. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McKleroy VS, Galbraith JS, Cummings B, Jones P, Harshbarger C, Collins C, Gelaude D, Carey JW, & Team A (2006). Adapting evidence–based behavioral interventions for new settings and target populations . AIDS Education and Prevention , 18 ( Suppl ), 59–73. 10.1521/aeap.2006.18.supp.59 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McNairy ML, & El-Sadr WM (2014). A paradigm shift: Focus on the HIV prevention continuum . Clinical Infectious Diseases , 59 ( Suppl 1 ), S12–S15. 10.1093/cid/ciu251 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Melnyk BM, & Fineout-Overholt E (2011). Evidence-based practice in nursing & healthcare: A guide to best practice . Lippincott Williams & Wilkins. [ Google Scholar ]
- Miller RL, Chiaramonte D, Strzyzykowski T, Sharma D, Anderson-Carpenter K, & Fortenberry JD (2019). Improving timely linkage to care among newly diagnosed HIV-infected youth: Results of SMILE . Journal of Urban Health , 96 ( 6 ), 845–855. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mitchell JW (2015). The use of technology to advance HIV prevention for couples . Current HIV/AIDS Reports , 12 ( 4 ), 516–522. 10.1007/s11904-015-0290-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, Tremblay C, Le Gall J-M, Cua E, Pasquet A, Raffi F, Pintado C, Chidiac C, Chas J, Charbonneau P, Delaugerre C, Suzan-Monti M, Loze B, Fonsart J, Peytavin G, …, & Delfraissy J-F (2015). On-demand preexposure prophylaxis in men at high risk for HIV-1 infection . New England Journal of Medicine , 373 ( 23 ), 2237–2246. 10.1056/NEJMoa1506273 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Muessig KE, Knudtson KA, Soni K, Larsen MA, Traum D, Dong W, Conserve DF, Leuski A, Artstein R, & Hightow-Weidman LB (2018). “I didn’t tell you sooner because I didn’t know how to handle it myself.” Developing a virtual reality program to support HIV-status disclosure decisions . Digital Culture & Education , 10 , 22–48. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Muessig KE, Nekkanti M, Bauermeister J, Bull S, & Hightow-Weidman LB (2015). A systematic review of recent smartphone, Internet and Web 2.0 interventions to address the HIV continuum of care . Current HIV/AIDS Reports , 12 ( 1 ), 173–190. 10.1007/s11904-014-0239-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, Barin B, Rooney JF, Mark D, Chetty P, Fast P, & Priddy FH (2012). Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers . PLoS One , 7 ( 4 ), e33103. 10.1371/journal.pone.0033103 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nadarzynski T, Morrison L, Bayley J, & Llewellyn C (2017). The role of digital interventions in sexual health . Sexually Transmitted Infections , 93 ( 4 ), 234–235. 10.1136/sextrans-2016-052926 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- National Institutes of Health. (2020). All of us research program . www.joinallofus.org/
- New York City Department of Health and Mental Hygiene. (2019). “On-Demand” dosing for PrEP: guidance for medical providers . Retrieved from https://www1.nyc.gov/assets/doh/downloads/pdf/ah/prep-on-demand-dosing-guidance.pdf .
- Niakan S, Mehraeen E, Noori T, & Gozali E (2017). Web and mobile based HIV prevention and intervention programs pros and cons: A review . Studies in Health Technology and Informatics , 236 , 319–327. https://umasslowell.idm.oclc.org/login?url5http://search.ebscohost.com/login.aspx?direct5true&db5cmedm&AN528508813&site5eds-live [ PubMed ] [ Google Scholar ]
- Njuguna N, Ngure K, Mugo N, Sambu C, Sianyo C, Gakuo S, Irungu E, Baeten J, & Heffron R (2016). The effect of human immunodeficiency virus prevention and reproductive health text messages on human immunodeficiency virus testing among young women in Rural Kenya: A pilot study . Sexually Transmitted Diseases , 43 ( 6 ), 353–359. 10.1097/OLQ.0000000000000450 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nosyk B, Zang X, Krebs E, Enns B, Min JE, Behrends CN, del Rio C, Dombrowski JC, Feaster DJ, Golden M, Marshall BDL, Mehta SH, Metsch LR, Pandya A, Schackman BR, Shoptaw S, Strathdee SA, Gebo KA, Kirk G, Montaner J, & Localized, H. I. V. M. S. G. (2020). Ending the HIV epidemic in the USA: An economic modelling study in six cities . The Lancet HIV , 7 ( 7 ), e491–e503. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- O’Byrne P, Macpherson P, Roy M, & Kitson C (2014). Overviewing a nurse-led, community-based HIV PEP program: Applying the extant literature in frontline practice . Public Health Nursing , 32 ( 3 ), 256–265. 10.1111/phn.12123 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Office of Infectious Disease and HIV/AIDS Policy. (2020). About ending the HIV epidemic: Plan for America . HIV.gov. Retrieved August 16, from https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview [ Google Scholar ]
- Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, Krakower D, Novak DS, Mimiaga MJ, & Mayer KH (2015). State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States . AIDS , 29 ( 7 ), 837–845. 10.1097/QAD.0000000000000622 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Page K, Tsui J, Maher L, Choopanya K, Vanichseni S, Mock PA, Celum C, & Martin M (2015). Biomedical HIV prevention including pre-exposure prophylaxis and opiate agonist therapy for women who inject drugs: State of research and future directions . Journal of Acquired Immune Deficiency Syndromes , 69 ( Suppl 2 ), S169–S175. 10.1097/QAI.0000000000000641 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pankow J, Willett J, Yang Y, Swan H, Dembo R, Burdon WM, Patterson Y, Pearson FS, Belenko S, & Frisman LK (2017). Evaluating fidelity to a modified NIATx process improvement strategy for improving HIV services in correctional facilities . The Journal of Behavioral Health Services & Research , 45 ( 2 ), 187–203. https://link.springer.com/content/pdf/10.1007/s11414-017-9551-1.pdf [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Payne-Foster P, Bradley ELP, Aduloju-Ajijola N, Yang X, Gaul Z, Parton J, Sutton MY, & Gaskins S (2018). Testing our FAITHH: HIV stigma and knowledge after a faith-based HIV stigma reduction intervention in the Rural South . AIDS Care , 30 ( 2 ), 232–239. https://www.tandfonline.com/doi/pdf/10.1080/09540121.2017.1371664?needAccess5true [ PubMed ] [ Google Scholar ]
- Peskin MF, Coyle KK, Anderson PM, Laris BA, Glassman JR, Franks HM, Thiel MA, Potter SC, Unti T, Edwards S, Johnson-Baker K, Cuccaro PM, Diamond P, Markham CM, Shegog R, Baumler ER, Gabay EK, & Emery ST (2019). Replication of it’s your game… Keep it real! In Southeast Texas . The Journal of Primary Prevention , 40 ( 3 ), 297–323. 10.1007/s10935-019-00549-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Peterson L, Taylor D, Roddy R, Belai G, Phillips P, Nanda K, Grant R, Clarke EEK, Doh AS, Ridzon R, Jaffe HS, & Cates W (2007). Tenofovir disoproxil fumarate for prevention of HIV infection in women: A phase 2, double-blind, randomized, placebo-controlled trial . PLoS Clinical Trials , 2 ( 5 ), e27. 10.1371/journal.pctr.0020027 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pitpitan EV, & Kalichman SC (2016). Reducing HIV risks in the places where people drink: Prevention interventions in alcohol venues . AIDS and Behavior , 20 , 119–133. 10.1007/s10461-015-1116-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Portillo CJ, Stringari-Murray S, Fox CB, Monasterio E, & Rose CD (2016). The HIV primary care workforce of tomorrow: The UCSF integrated HIV/AIDS primary care capacity nurse practitioner program . Journal of the Association of Nurses in AIDS Care , 27 ( 3 ), 214–222. https://journals.lww.com/janac/Fulltext/2016/05000/The_HIV_Primary_Care_Workforce_of_Tomorrow__The.3.aspx [ PubMed ] [ Google Scholar ]
- Powell BJ, McMillen JC, Proctor EK, Carpenter CR, Griffey RT, Bunger AC, Glass JE, & York JL (2012). A compilation of strategies for implementing clinical innovations in health and mental health . Medical Care Research and Review , 69 ( 2 ), 123–157. 10.1177/1077558711430690 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Psomas CK, Barber TJ, Rutsaert S, & Kinloch-de Loës S (2017). Highlights from the 9th IAS Conference on HIV Science, 23–26 July 2017, Paris, France . Journal of Virus Eradication , 3 ( 4 ), 242–249. https://pubmed.ncbi.nlm.nih.gov/29057090 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ramos SR (2017). User-centered design, experience, and usability of an electronic consent user interface to facilitate informed decision-making in an HIV clinic . Computers, Informatics, Nursing: CIN , 35 ( 11 ), 556–564. 10.1097/CIN.0000000000000356 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ramos SR, Warren R, Shedlin M, Melkus G, Kershaw T, & Vorderstrasse A (2019). A framework for using ehealth interventions to overcome medical mistrust among sexual minority men of color living with chronic conditions . Behavioral Medicine , 45 ( 2 ), 166–176. 10.1080/08964289.2019.1570074 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rhodes SD, Alonzo J, Mann L, Song EY, Tanner AE, Arellano JE, Rodriguez-Celedon R, Garcia M, Freeman A, Reboussin BA, & Painter TM (2017). Small-group randomized controlled trial to increase condom use and HIV testing among Hispanic/Latino gay, bisexual, and other men who have sex with men . American Journal of Public Health , 107 ( 6 ), 969–976. 10.2105/AJPH.2017.303814 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rhodes SD, Leichliter JS, Sun CJ, & Bloom FR (2016). The HoMBReS and HoMBReS Por un Cambio interventions to reduce HIV disparities among immigrant Hispanic/Latino men . MMWR Supplements , 65 ( 1 ), 51–56. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rhodes SD, McCoy TP, Tanner AE, Stowers J, Bachmann LH, Nguyen AL, & Ross MW (2016). Using social media to increase HIV testing among gay and bisexual men, other men who have sex with men, and transgender persons: Outcomes from a randomized community trial . Clinical Infectious Diseases , 62 ( 11 ), 1450–1453. 10.1093/cid/ciw127 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rodríguez Vargas B, Sánchez-Rubio Ferrández J, Garrido Fuentes J, Velayos R, Morillo Verdugo R, Sala Piñol F, Onteniente González A, & Rodríguez Sagrado MÁ (2019). Usability and acceptability of a comprehensive HIV and other sexually transmitted infections prevention app . Journal of Medical Systems , 43 ( 6 ), 175. 10.1007/s10916-019-1323-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rowniak S, Ong-Flaherty C, Selix N, & Kowell N (2017). Attitudes, beliefs, and barriers to PrEP among trans men . AIDS Education and Prevention , 29 ( 4 ), 302–314. http://dx.doi.org/101521aeap2017294302 [ PubMed ] [ Google Scholar ]
- Ruiz MS, O’Rourke A, & Allen ST (2016). Impact evaluation of a policy intervention for HIV prevention in Washington, DC . AIDS and Behavior , 20 ( 1 ), 22–28. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- San Francisco Department of Public Health. (2019). San Francisco Department of Public Health (SFDPH) statement on non-daily (2-1-1) emtricitabine/tenofovir dosing for PrEP . Retrieved from http://www.gettingtozerosf.org/wp-content/uploads/2018/11/HIVUpdate_02122019_v2.pdf
- Schnall R, Bakken S, Rojas M, Travers J, & Carballo-Dieguez A (2015). mHealth technology as a persuasive tool for treatment, care and management of persons living with HIV . AIDS and Behavior , 19 ( 2 ), 81–89. 10.1007/s10461-014-0984-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schnall R, Rojas M, Bakken S, Brown W, Carballo-Dieguez A, Carry M, … & Travers J (2016). A user-centered model for designing consumer mobile health (mHealth) applications (apps) . Journal of biomedical informatics , 60 , 243–251. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schnall R, Liu J, Mohr DC, Bakken S, Hirshfield S, Siegel K, Stonbraker S, Cho H, Iribarren S, & Voss J (2019). Multi-modal methodology for adapting digital health tools to new populations: Adaptation of the video information provider (VIP) for persons living with HIV with HIV-associated non-AIDS (HANA) conditions . Studies in Health Technology and Informatics , 264 , 1347–1351. 10.3233/SHTI190446 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shain RN, Piper JM, Newton ER, Perdue ST, Ramos R, Champion JD, & Guerra FA (1999). A randomized, controlled trial of a behavioral intervention to prevent sexually transmitted disease among minority women . New England Journal of Medicine , 340 ( 2 ), 93–100. 10.1056/NEJM199901143400203 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shelley G, Williams W, Uhl G, Hoyte T, Eke A, Wright C, Rebchook G, Pollack L, Bell K, Wang Y, Cheng Q, & Kegeles SM (2017). An evaluation of Mpowerment on individual-level HIV risk behavior, testing, and psychosocial factors among young MSM of color: The monitoring and evaluation of MP (MEM) project . AIDS Education and Prevention , 29 ( 1 ), 24–37. [ PubMed ] [ Google Scholar ]
- Shieh E, Marzinke MA, Fuchs EJ, Hamlin A, Bakshi R, Aung W, Breakey J, Poteat T, Brown T, Bumpus NN, & Hendrix CW (2019). Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking oestrogen when compared to cisgender men . Journal of the International AIDS Society , 22 ( 11 ), e25405. 10.1002/jia2.25405 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siegler AJ, Brock JB, Hurt CB, Ahlschlager L, Dominguez K, Kelley CF, & Mena LA (2019). An electronic pre-exposure prophylaxis initiation and maintenance home care system for nonurban young men who have sex with men: Protocol for a randomized controlled trial . JMIR Research Protocols , 8 ( 6 ), e13982. 10.2196/13982 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siegler AJ, Steehler K, Sales JM, & Krakower DS (2020). A review of HIV pre-exposure prophylaxis streamlining strategies . Current HIV/AIDS Reports , 17 , 643–653. 10.1007/s11904-020-00528-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siguier M, Mera R, Pialoux G, Ohayon M, Cotte L, Valin N, Ghosn J, Cua E, Pintado C, Chas J, Barriere G, Durand F, & Molina JM (2019). First year of pre-exposure prophylaxis implementation in France with daily or on-demand tenofovir disoproxil fumarate/emtricitabine . Journal of Antimicrobial Chemotherapy , 74 ( 9 ), 2752–2758. 10.1093/jac/dkz220 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Solomon J, Card JJ, & Malow RM (2006). Adapting efficacious interventions: Advancing translational research in HIV prevention . Evaluation & the Health Professions , 29 ( 2 ), 162–194. 10.1177/0163278706287344 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Springer SA, Di Paola A, Azar MM, Barbour R, Biondi BE, Desabrais M, Lincoln T, Skiest DJ, & Altice FL (2018). Extended-release naltrexone improves viral suppression among incarcerated persons living with HIV with opioid use disorders transitioning to the community: Results of a double-blind, placebo-controlled randomized trial . Journal of Acquired Immune Deficiency Syndromes , 78 ( 1 ), 43–53. 10.1097/QAI.0000000000001634 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stevens RC, Brawner BM, Kranzler E, Giorgi S, Lazarus E, Abera M, Huang S, & Ungar L (2020). Exploring substance use tweets of youth in the United States: Mixed methods study . JMIR Public Health Surveillance , 6 ( 1 ), e16191. 10.2196/16191 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stevens R, Gilliard-Matthews S, Dunaev J, Todhunter-Reid A, Brawner B, & Stewart J (2017). Social media use and sexual risk reduction behavior among minority youth: Seeking safe sex information . Nursing Research , 66 ( 5 ), 368–377. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sullivan PS, Driggers R, Stekler JD, Siegler A, Goldenberg T, McDougal SJ, Caucutt J, Jones J, & Stephenson R (2017). Usability and acceptability of a mobile comprehensive HIV prevention app for men who have sex with men: A pilot study . JMIR mHealth and uHealth , 5 ( 3 ), e26. 10.2196/mhealth.7199 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sun CJ, Mann L, Eng E, Downs M, & Rhodes SD (2015). Once a navegante, always a navegante: Latino men sustain their roles as lay health advisors to promote general and sexual health to their social network . AIDS Education and Prevention , 27 ( 5 ), 465–473. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Theall KP, Fleckman J, & Jacobs M (2015). Impact of a community popular opinion leader intervention among African American adults in a southeastern united states community . AIDS Education and Prevention , 27 ( 3 ), 275–288. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, … & Brooks JT (2012). Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana . New England Journal of Medicine , 367 ( 5 ), 423–434. 10.1056/NEJMoa1110711 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Threats M & Bond K (2021). HIV information acquisition and use among young Black men who have sex with men who use the internet: a mixed methods study . Journal of Medical Internet Research , 23 ( 5 ), e22986. https://pubmed.ncbi.nlm.nih.gov/33960953 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tobin K, Davey-Rothwell MA, Nonyane BAS, Knowlton A, Wissow L, & Latkin CA (2017). RCT of an integrated CBT-HIV intervention on depressive symptoms and HIV risk . PLoS One , 12 ( 12 ), e0187180. 10.1371/journal.pone.0187180 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Towe VL, Wiewel EW, Zhong Y, Linnemayr S, Johnson R, & Rojas J (2019). A randomized controlled trial of a rapid re-housing intervention for homeless persons living with HIV/AIDS: Impact on housing and HIV medical outcomes . AIDS and Behavior , 23 ( 9 ), 2315–2325. 10.1007/s10461-019-02461-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Treston C (2019). Ending the HIV epidemic: A plan for America—Nurses needed . Journal of the Association of Nurses in AIDS Care , 30 ( 3 ), 379–380. 10.1097/JNC.0000000000000091 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tung EL, Thomas A, Eichner A, & Shalit P (2018). Implementation of a community pharmacy-based pre-exposure prophylaxis service: A novel model for pre-exposure prophylaxis care . Sexual Health , 15 ( 6 ), 556–561. 10.1071/sh18084 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Underwood C, Hendrickson Z, Van Lith LM, Lengwe Kunda JE, & Mallalieu EC (2014). Role of community-level factors across the treatment cascade: A critical review . Journal of Acquired Immune Deficiency Syndromes , 66 , S311–S318. 10.1097/qai.0000000000000234 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- U.S. Centers for Disease Control and Prevention, & U.S. Public Health Service. (2018). Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update: A clinical practice guideline . https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
- U.S. Department of Health and Human Services. (2019). Ending the HIV epidemic: a plan for America . Retrieved from: https://www.cdc.gov/endhiv/docs/ending-HIV-epidemic-overview-508.pdf
- U.S. Food & Drug Administration. (2019, October 3). FDA approves second drug to prevention HIV infection as part of ongoing efforts to end the HIV epidemic . Retrieved August 3, from https://www.fda.gov/news-events/press-announcements/fda-approves-second-drug-prevent-hiv-infection-part-ongoing-efforts-end-hiv-epidemic
- Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak’Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, … & Taylor D (2012). Preexposure prophylaxis for HIV infection among African women . New England Journal of Medicine , 367 ( 5 ), 411–422. 10.1056/NEJMoa1202614 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Velloza J, Ngure K, Kiptinness C, Quame-Amaglo J, Thuo N, Dew K, Kimani M, Gakuo S, Unger JA, Kolko B, Baeten JM, Celum C, Mugo N, & Heffron R (2019). A clinic-based tablet application to support safer conception among HIV serodiscordant couples in Kenya: Feasibility and acceptability study . MHealth , 5 , 4. 10.21037/mhealth.2019.01.04 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Villarruel AM, Jemmott JB, Jemmott LS, & Ronis DL (2007). Predicting condom use among sexually experienced Latino adolescents . Western Journal of Nursing Research , 29 ( 6 ), 724–738. 10.1177/0193945907303102 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ware NC, Pisarski EE, Tam M, Wyatt MA, Atukunda E, Musiimenta A, Bangsberg DR, & Haberer JE (2016). The Meanings in the messages: How SMS reminders and real-time adherence monitoring improve antiretroviral therapy adherence in rural Uganda . AIDS , 30 ( 8 ), 1287–1293. 10.1097/QAD.0000000000001035 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Werb D, Garfein R, Kerr T, Davidson P, Roux P, Jauffret-Roustide M, Auriacombe M, Small W, & Strathdee SA (2016). A socio-structural approach to preventing injection drug use initiation: Rationale for the PRIMER study . Harm Reduction Journal , 13 ( 1 ), 25. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Whiteley LB, Brown LK, Curtis V, Ryoo HJ, & Beausoleil N (2018). Publicly available internet content as a HIV/STI prevention intervention for urban youth . Journal of Primary Prevention , 39 ( 4 ), 361–370. 10.1007/s10935-018-0514-y [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Willie T, Kershaw T, Campbell JC, & Alexander KA (2017). Intimate partner violence and PrEP acceptability among low-income, young Black women: Exploring the mediating role of reproductive coercion . AIDS and Behavior , 21 ( 8 ), 2261–2269. 10.1007/s10461-017-1767-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wingood GM, DiClemente RJ, Mikhail I, Lang DL, McCree DH, Davies SL, Hardin JW, Hook EW, & Saag M (2004). A randomized controlled trial to reduce HIV transmission risk behaviors and sexually transmitted diseases among women living with HIV: The WiLLOW Program . Journal of Acquired Immune Deficiency Syndromes , 37 ( Suppl 2 ), S58–S67. 10.1097/01.qai.0000140603.57478.a9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wohl DA, Golin CE, Knight K, Gould M, Carda-Auten J, Groves JS, Napravnik S, Cole SR, White BL, Fogel C, Rosen DL, Mugavaro MJ, Pence BW, & Flynn PM (2017). Randomized controlled trial of an intervention to maintain suppression of HIV viremia after prison release: The imPACT Trial . Journal of Acquired Immune Deficiency Syndromes , 75 ( 1 ), 81–90. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5443668/pdf/nihms853764.pdf [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wood BR, Mann MS, Martinez-Paz N, Unruh KT, Annese M, Spach DH, Scott JD, & Stekler JD (2018). Project ECHO: Telementoring to educate and support prescribing of HIV pre-exposure prophylaxis by community medical providers . Sexual Health , 15 ( 6 ), 601–605. [ PubMed ] [ Google Scholar ]
- Wootton AR, Legnitto DA, Gruber VA, Dawson-Rose C, Neilands TB, Johnson MO, & Saberi P (2019). Telehealth and texting intervention to improve HIV care engagement, mental health and substance use outcomes in youth living with HIV: A pilot feasibility and acceptability study protocol . BMJ Open , 9 ( 7 ), e028522. 10.1136/bmjopen-2018-028522 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wray T, Chan PA, Simpanen E, & Operario D (2017). eTEST: Developing a smart home HIV testing kit that enables active, real-time follow-up and referral after testing . JMIR mHealth and uHealth , 5 ( 5 ), e62. 10.2196/mhealth.6491 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yager JL, & Anderson PL (2020). Pharmacology and drug interactions with HIV PrEP in transgender persons receiving gender affirming hormone therapy . Expert Opinion on Drug Metabolism & Toxicology , 16 ( 6 ), 463–474. 10.1080/17425255.2020.1752662 [ PubMed ] [ CrossRef ] [ Google Scholar ]

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Office of AIDS Research
- HIV Policy and Research
- Research Priorities
- NIH Strategic Plan for HIV and HIV-Related Research
- NIH HIV Research Budget
- NIH HIV Research Priority Areas
- Reduce the Incidence of HIV
- Develop Next-Generation HIV Therapies
Research Toward HIV Cure
- Address HIV-Associated Comorbidities, Coinfections, & Complications
- Cross-Cutting Areas

Viral latency and sanctuaries
Latent HIV reservoirs—small amounts of HIV that persist in people taking ART—present a significant challenge to finding a cure for HIV. Latent reservoirs remain in people with HIV when HIV becomes part of the body’s DNA in infected cells. Additionally, reservoirs of HIV can be found in certain “sanctuary” sites in the body that allow the virus to hide and be protected from both the immune system and ART. To cure HIV, the NIH supports studies to develop novel approaches and treatments that target these HIV reservoirs.
Sustained viral remission and viral eradication

Current science suggests that the path to an HIV cure involves first achieving sustained viral remission without ART. This is called sustained ART-free viral remission or a functional cure. For sustained ART-free viral remission, infectious virus must remain undetectable by sensitive testing methods for a long time without treatment. One research aim will be to prolong the time between treatments to be measured eventually not in weeks, but in months or even years. The NIH supports research into treatments leading to sustained ART-free viral remission . New cure-inducing treatments must be as safe, effective, and available for widespread use as are current-day ART regimens.
Viral eradication—eliminating the virus entirely—is the more challenging, longer-term goal.
Research Strategies
The NIH supports research to better understand how the HIV reservoir forms, persists, and reactivates, as well as investigations to develop new cure treatment strategies targeting HIV reservoirs.
A range of biomarkers and techniques, including single-cell and imaging technologies, are being studied to determine how to identify and describe the HIV reservoir. These techniques also are being used to better understand mechanisms of viral reactivation from latently infected cells.
Experimental treatments in development include therapeutic vaccines, genetically engineered immune cells that are resistant to HIV infection, drugs that reactivate latent HIV to make the virus visible to the immune system, cure-inducing immunotherapies, and interventions to permanently silence HIV in infected cells.
The search for an HIV cure involves important behavioral and social processes that complement the domains of biomedicine. BSSR in HIV cure research is focused on important aspects such as: counseling and support interventions to address the psychosocial needs and concerns of study participants related to analytical treatment interruptions (ATIs); risk reduction in the course of ATI study participation; motivation, acceptability, and decision‐making processes of potential study participants; how cure affects the identity and social position of people with HIV; and the scalability of a proven cure strategy in the context of further advances in HIV prevention and treatment.

The NIH is leveraging resources toward an HIV cure through several public-private partnerships. NIH small business awards enable companies to help foster a diverse pipeline of experimental treatments in development. The combined support of government, industry, and nongovernmental foundations is fostering the expansion of a talented scientific workforce dedicated to advancing HIV cure research.
OAR scientist Dr. Paul Sato coordinates Research Toward an HIV Cure .
This page last reviewed on September 8, 2022
After decades of failures, researchers have renewed hopes for an effective HIV vaccine
The world needs an HIV vaccine if it ever hopes to beat a virus that still infects over 1 million people a year and contributes to hundreds of thousands of deaths.
Despite 20 years of failures in major HIV vaccine trials — four this decade alone — researchers say recent scientific advances have likely, hopefully, put them on the right track to develop a highly effective vaccine against the insidious virus.
But probably not until the 2030s.
“An effective vaccine is really the only way to provide long-term immunity against HIV, and that’s what we need,” Dr. Julie McElrath, the director of the vaccine and infectious disease division at the Fred Hutchinson Cancer Center in Seattle, said Monday at the Conference on Retroviruses and Opportunistic Infections in Denver.
All current HIV vaccine action is in the laboratory, animal studies or very early human trials.
Researchers at the retrovirus conference presented favorable results from two HIV vaccine studies. One found that a modification to the simian version of HIV spurred production of what are known as broadly neutralizing antibodies against the virus in monkeys. Another showed promise in the effort to coax the immune system’s B cells to make the powerful antibodies in humans.
“These trials illustrate as a proof of concept that we can train the immune system. But we need to further optimize it and test it in clinical trials,” Karlijn van der Straten, a Ph.D. student at the Academic Medical Center at Amsterdam University, who presented the human study, said at a news conference Monday.
Still, the scrappy scientists in this field face a towering challenge. HIV is perhaps the most complex pathogen ever known.
“The whole field has learned from the past,” said William Schief, who leads Moderna’s HIV vaccine efforts. “We’ve learned strategies that don’t work.”
The cost has already been immense. Nearly $17 billion was spent worldwide on HIV -vaccine research from 2000 to 2021. Nearly $1 billion more is spent annually, according to the Joint United Nations Program on HIV/AIDS and the nonprofit HIV group AVAC.
“Maintaining the funding for HIV vaccines right now is really important,” said Dr. Nina Russell, who directs HIV research at the Bill & Melinda Gates Foundation. She pointed to the field’s own “progress and the excitement” and to how “HIV vaccine science and scientists continue to drive innovation and science that benefits other infectious diseases and global health in general.”
Case in point: Covid. Thanks to HIV research, the mRNA vaccine technology was already available in 2020 to speed a coronavirus vaccine to market.
Why the HIV vaccine efficacy trials failed
In strong contrast to Covid, the HIV vaccine endeavor has spanned four decades. Only one of the nine HIV vaccine trials have shown efficacy: a trial conducted in Thailand and published in 2009 that reported a modest 31% reduction in HIV risk.
HIV vaccine researchers subsequently spent years seeking to retool and improve that vaccine strategy, leading to a series of trials that launched in the late 2010s — only to fail.
Researchers have concluded those latest trials were doomed because, aside from prompting an anti-HIV response based in immune cells, they only drove the immune system to produce what are known as non-neutralizing antibodies. Those weapons just weren’t strong enough for such a fearsome foe.
Preventing HIV through vaccination remains a daunting challenge because the immune system doesn’t naturally mount an effective defense against the virus, as it does with so many other vaccine-preventable infections, including Covid. An HIV vaccine must coax from the body a supercharged immune response with no natural equivalent.
That path to victory is based on a crucial caveat: A small proportion of people with HIV do produce what are known as broadly neutralizing antibodies against the virus. They attack HIV in multiple ways and can neutralize a swath of variants of the virus.
Those antibodies don’t do much apparent good for people who develop them naturally, because they typically don’t arise until years into infection. HIV establishes a permanent reservoir in the body within about a week after infection, one that their immune response can’t eliminate. So HIV-positive people with such antibodies still require antiretroviral treatment to remain healthy.
Researchers believe that broadly neutralizing antibodies could prevent HIV from ever seeding an infection, provided the defense was ready in advance of exposure. A pair of major efficacy trials, published in 2021 , demonstrated that infusions of cloned versions of one such antibody did, indeed, protect people who were exposed to certain HIV strains that are susceptible to that antibody.
However, globally, those particular strains of the virus comprise only a small subset of all circulating HIV. That means researchers can’t simply prompt a vaccine to produce that one antibody and expect it to be effective. Importantly, from this study they got a sense of what antibody level would be required to prevent infection.
It’s a high benchmark, but at least investigators now have a clearer sense of the challenge before them.
Also frustrating the HIV vaccine quest is that the virus mutates like mad. Whatever spot on the surface of the virus that antibodies target might be prone to change through mutation, thus allowing the virus to evade their attack. Consequently, researchers search for targets on the virus’ surface that aren’t highly subject to mutation.
Experts also believe warding off the mutation threat will require targeting multiple sites on the virus. So researchers are seeking to develop a portfolio of immune system prompts that would spur production of an array of broadly neutralizing antibodies.
Prompting the development of such antibodies requires a complex, step-by step process of coaxing the infection-fighting B cells, getting them to multiply and then guiding their maturation into potent broadly neutralizing antibody-producing factories.
HIV vaccine development ‘in a better place’
Dr. Carl Dieffenbach, the head of the AIDS division at the National Institute of Allergy and Infectious Diseases, said numerous recent technological advances — including mRNA, better animal models of HIV infection and high-tech imaging technology — have improved researchers’ precision in designing, and speed in producing, new proteins to spur anti-HIV immune responses.
Global collaboration among major players is also flourishing, researchers said. There are several early-stage human clinical trials of HIV-vaccine components underway.
Three mRNA- based early human trials of such components have been launched since 2022. Among them, they have been led or otherwise funded by the global vaccine research nonprofit group IAVI, Fred Hutch, Moderna, Scripps Research, the Gates Foundation, the National Institutes of Health, the U.S. Agency for International Development, and university teams. More such trials are in the works.
On Friday, Science magazine reported concerning recent findings that among the three mRNA trials, a substantial proportion of participants — 7% to 18%, IAVI said in a statement — experienced skin-related symptoms following injections, including hives, itching and welts.
IAVI said in its statement that it and partners are investigating the HIV trials’ skin-related outcomes, most of which were “mild or moderate and managed with simple allergy medications.”
Researchers have shown success in one of those mRNA trials in executing a particular step in the B-cell cultivation process.
That vaccine component also generated “helper” CD4 cells primed to combat HIV. The immune cells are expected to operate like an orchestra conductor for the immune system, coordinating a response by sending instructions to B cells and scaling up other facets of an assault on HIV.
A complementary strategy under investigation seeks to promote the development of “killer” CD8 cells that might be primed to kill off any immune cells that the antibodies failed to save from infection.
Crucially, investigators believe they are now much better able to discern top vaccine component candidates from the duds. They plan to spend the coming years developing such components so that when they do assemble the most promising among them into a multi-pronged vaccine, they can be much more confident of ultimate success in a trial.
“An HIV vaccine could end HIV,” McElrath said at the Denver conference. “So I say, ‘Let’s just get on with it.”
Dr. Mark Feinberg, president and CEO of IAVI, suggested that the first trial to test effectiveness of the vaccine might not launch until 2030 or later.
Even so, he was bullish.
“The field of HIV vaccine development is in a better place now than it’s ever been,” he said.
Benjamin Ryan is independent journalist specializing in science and LGBTQ coverage. He contributes to NBC News, The New York Times, The Guardian and Thomson Reuters Foundation and has also written for The Washington Post, The Nation, The Atlantic and New York.
- Español (Spanish)
DRUG DATABASE
Services Locator

Personalize Your Experience
Log in or create an account for a personalized experience based on your selected interests.
Already have an account? Log In
Free standard shipping is valid on orders of $45 or more (after promotions and discounts are applied, regular shipping rates do not qualify as part of the $45 or more) shipped to US addresses only. Not valid on previous purchases or when combined with any other promotional offers.
Register for an enhanced, personalized experience.
Receive free access to exclusive content, a personalized homepage based on your interests, and a weekly newsletter with topics of your choice.
Home / Innovation & Research / The innovative research behind HIV/AIDS treatment
The innovative research behind HIV/AIDS treatment
Please login to bookmark.
Username or Email Address
Remember Me

It’s been 40 years since the release of the first scientific report describing acquired immune deficiency syndrome (AIDS). Thanks to innovative research, scientists learned how the HIV virus that causes AIDS replicates and how the immune system responds to the virus. Today, many people with HIV take just one pill a day to suppress the virus, and treatment is continuing to evolve.
In this video, Dr. Stacey Rizza , Mayo Clinic infectious disease physician and HIV researcher, explains how dedicated innovative science contributed to where we are today and what scientists are working on for the future.
What did the early research find?
Because of truly dedicated innovative science, within a few years, the scientific community figured out that AIDS was due to HIV. It then took a few years to figure out how to test for that virus. Several years later, the scientific community was able to quantitate how much virus was in a person’s blood. During all this time, truly innovative research into how the virus replicates and how the immune system responds to the virus allowed bio pharmacy companies to develop what we call anti-retroviral drugs or medications to slow down the viral replication. How has medication to treat HIV evolved?
The first drug approved for HIV was in 1987, which was AZT (now known as zidovudine). At that time, it was the fastest drug ever approved by the FDA (Food and Drug Administration) and started the fast-track mechanism through the FDA.
Then several other drugs within that same class were approved in the early 1990s. In late 1995, very early 1996, the first HIV protease inhibitors were approved. At that point, it was possible to combine three different medications from two different classes and completely suppress the HIV replication.
In the last 20 years, we’ve gone from people taking multiple medicines with lots of side effects to many of my patients with HIV now take a single pill a day. That’s a combination of medicines coformulated into one pill a day that’s extremely well-tolerated and completely suppresses their virus. We know it does not eliminate the virus. If they were to stop taking that medicine, the virus would come back. But we now have a handful of people in the world who have been what we called functionally cured of HIV, meaning they’ve gone through some research protocols that eliminated the reservoir of HIV in their body.
The new drugs are so effective in people who have fully suppressed virus that many only need to use two medications to maintain HIV treatment and control. New research is investigating ways to deliver the medications differently, such as a shot that lasts several months, or maybe someday even implantable medication delivery mechanisms so that people don’t have to take the pill every day. It is very exciting that HIV therapy is moving that direction.
Why isn’t there a cure for HIV?
The reason why it is so difficult to cure HIV is that once HIV infects a person’s body, it integrates into the host genome of several cell types. Those cells then hide in any of the lymphoid tissue, such as the lymph nodes, the liver and the spleen. And they lay there as what we call “latent” or “hiding”, as long as the person is on HIV therapy. Anytime a virus does leave a cell, it gets taken care of by HIV therapy. But if the infected individual stops the HIV therapy, that latent virus will come back. To cure HIV, you have to eliminate those hiding viruses in the cells or that latent viral reservoir, which is the term. There are many ways you can approach eliminating the reservoir.
Where is the research now?
One of the more popular ways that have been investigated is something called — and there are many different terms for it — “prime, shock, and kill” or “kick, and kill”, which is essentially giving medications that first wake the virus up from latency and then find ways to make the cells that have the virus susceptible to dying. When the virus is awake, and the cell is susceptible to dying, it kills itself but does not kill any other cells in the body.
Essentially, it specifically targets the HIV-infected cells and eliminates them without hurting anything else. This new science is exciting. It’s getting closer and closer to understanding how to do this effectively. And if you can do that with oral medications rather than fancy therapies like gene therapy or bone marrow transplant, it’s scalable to large parts of the world, and you can touch millions of people that way. That’s where the area of research is on how to make those hiding cells wake up, how to make them sensitive to die, and how to target just the HIV-infected cell.
Will we see a vaccine for HIV?
HIV has been a very hard vaccine to develop. In the world of viruses, vaccines fall into one of three buckets. They fall into the bucket where they respond to antibodies induced by the vaccine, and the vaccines are outstanding. Such viruses include polio, mumps, and lucky for us, SARS-CoV-2. Then we have the second category, like the influenza vaccine, which is about 60% effective. It certainly saves lives and makes a difference, but it’s not perfect. And then we have the third bucket, which quite frankly is the vast majority of viruses that infect humans. And HIV is in that category, where simply forming an antibody to the virus is not adequate to prevent infection. You have to do very sophisticated engineering to induce T cell effects, as well as innate effects and antibody effects. Even then, sometimes it’s very hard to decide what is the part of the virus to target. After decades, and billions of dollars of research, we’re still not there for HIV. There have been many approaches of how to do this science. Many different scientific delivery mechanisms, many different areas of the viruses targeted, many different parts of the immune system targeted, and so far, none of them have been effective at preventing HIV infection.
What needs to happen next?
We still need to slow down the number of people getting infected through good public health measures and good education to stop the HIV epidemic. We still need to get more people who are infected on therapy.
We know we can do it with public health measures. But we also need to find out more about how we eliminate that reservoir and get people cured of the virus in a simple and effective way so that we can cure more people. And the last major hurdle we have is to develop an effective vaccine. We still don’t have a vaccine that can prevent infection, a preventive vaccine, or a therapeutic vaccine where you give it to people who already have the virus that can help them control the infection. A huge amount of research has happened, but we’re still not there yet.
This article originally appeared on Mayo Clinic News Network.

Relevant reading
The Nurses of Mayo Clinic
Spanning more than a century of dedication and service, this hardcover book presents the spirit of nursing at Mayo Clinic. Set against the background of national and world events and mirroring the remarkable progress of medicine, also included are many letters and photographs that are made public for the first…

Discover more Innovation & Research content from articles, podcasts, to videos.
You May Also Enjoy

by Alfredo Quiñones-Hinojosa, M.D.

by Fred Appelbaum, M.D.

by David Blistein, Ken Burns

Privacy Policy
We've made some updates to our Privacy Policy. Please take a moment to review.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- ADVERTISEMENT FEATURE Advertiser retains sole responsibility for the content of this article
HIV: Progress and future challenges in treatment, prevention and cure
- Diana Brainard ,
- Tomas Cihlar ,
- Romas Geleziunas &
- Devi SenGupta
- Diana Brainard
- Tomas Cihlar
- Romas Geleziunas
Produced by
Advances in care over the last 30 years have helped transform HIV from a fatal disease into a chronic, manageable condition for many people. 1 Innovations in treatment and access are at the core of this progress. The advent of antiretroviral therapy (ART), once-daily single tablet regimens (STRs) with three medications combined into one pill, and many subsequent improvements in STRs combining efficacy and long-term safety represent some of the most significant milestones that have helped bring radical change in the outlook for people living with HIV.
Progress in treatment has been transformative, but significant gaps in care still remain. HIV continues to be a major global public health issue. In the United States, the South accounts for seven of the top ten states and nine of the top ten metropolitan areas with the highest rates of new infections. This is pointed evidence that the epidemic is ongoing for some communities. 2 , 3 Globally, it’s estimated that more than 36 million people are living with HIV with acute challenges faced by Sub-Saharan Africa and many low- and middle-income countries. 4 With the goal of reducing the individual and societal burden of HIV, prevention of new infections is as important as treating those who are living with HIV.
At Gilead, we believe that anyone living with or at risk of HIV should benefit from the latest treatment innovations and have access to medicines. For almost 30 years, we have been at the forefront of the fight against HIV by leading advances in treatment, prevention and access. Our experience, dedication, and infrastructure are helping us move forward as we continue to focus on and make major investments in further expanding the options for HIV treatment and prevention. Equally as important, our close connections with the field, built over decades of collaborative research and development, enable us to help advance preclinical and clinical programs with the goal of achieving a functional cure for HIV.
Reducing Pill Burden with Single Tablet Regimens
In the early days of the HIV epidemic, high pill burden made successful adherence to treatment challenging. 5 Selective non-adherence to a regimen increases the likelihood of developing resistance, which could limit future treatment options. 6 STRs have revolutionized care by simplifying dosing and their impact has been profound and lasting. Today, clinical treatment guidelines from the US Department of Health and Human Services (DHHS) include certain STRs as recommended initial treatment regimen options for most people living with HIV. According to DHHS, factors that should guide the selection of a treatment regimen include virologic efficacy, toxicity, pill burden, dosing frequency, drug-drug interaction potential, resistance test results, comorbid conditions, access, and cost. 7
Despite the progress made in improving the efficacy and safety of HIV treatments, there are people with severely limited treatment options due to resistance to multiple ART classes. 6 They rely on complex treatment regimens, sometimes involving injectable medications in combination with multiple pills several times a day. 8 This type of complexity further increases the chance of treatment failure. Thus, we continue to strive to develop new agents that are active against resistant variants of HIV and target novel mechanisms of action, in order to provide simpler and more efficacious treatment options to all people living with HIV, irrespective of their prior treatment history. Our goal is to continue pursuing further ART innovations that enable STRs to reach beyond first and second line therapy options.
Researching Care with Long-Acting ART
Efforts to continue enhancing care will be critical to further curbing the HIV epidemic. Long-acting formulations with the potential to be dosed weekly, monthly, or even less frequently may help facilitate treatment adherence, a strong predictor of treatment success and health outcomes. 9 To become suitable for long-acting administration, new agents need to fit criteria different from those applied to daily oral ART. Novel regimens must match the high efficacy and safety observed with currently approved HIV treatments and do so with administration that is ideally minimally painful and can be performed outside a doctor’s office.
Long-acting antiretrovirals have already been identified among integrase and reverse transcriptase inhibitors, but additional drug targets are needed to help realize the full potential of long-acting ART for a range of patients. 10 Capsid, a multimeric shell present in infectious viral particles, is essential for virus replication as it plays crucial roles in both early and late stages of the viral life cycle. HIV capsid inhibitors represent a potential new class of antiretrovirals with high in vitro potency and very slow metabolic clearance leading to a prolonged half-life in in-vivo preclinical models. 11 Importantly, treatment failure due to circulating or archived pre-existing resistance is unlikely in the setting of novel therapeutic targets. Optimized combinations of multiple genetically engineered broadly neutralizing anti-HIV antibodies might also represent a new class of agents with long-acting potential. 12
Pioneering Prevention Options
Prevention methods and practices are essential tools in the fight against HIV. The innovation of pre-exposure prophylaxis (PrEP), an approach that involves daily antiretroviral medication in combination with safer sex practices to reduce the chance of acquiring HIV in individuals at risk for HIV, has been widely accepted by community advocates and is included in World Health Organization (WHO) and US Centers for Disease Control (CDC) clinical guidelines as part of a comprehensive prevention strategy. 13 , 14 Data are emerging to suggest the rate of new HIV infections can be dramatically reduced when PrEP and Treatment as Prevention (TasP) approaches are broadly adopted. 15
Since the introduction of PrEP as a prevention strategy, there have been ongoing initiatives across industry, government and community to help drive successful implementation, ranging from building awareness and improving persistence among communities disproportionately impacted by HIV to encouraging appropriate use through daily adherence. Despite an increase in PrEP uptake over the last five years among men who have sex with men, use among women has remained relatively low. 16 , 17 The reasons are likely multifactorial; however, providing additional PrEP treatment options, such as long-acting agents, could have an impact on engaging women at risk for HIV. 18 In this context, innovations in long-acting ART have the potential to be extended from treatment to prevention.
Continuing Innovation in Treatment and Prevention Access
Today’s access challenges will require continued evaluation and innovation. Diligent and responsive programs that regularly refine their approach to access scale-up will have the most success.
In the US, this requires industry leadership and close coordination with community partners as well as federal, state, and local policymakers. Early stage diagnosis and treatment initiation, connection to healthcare, and community support are key factors. Gilead’s FOCUS (Frontlines of Communities in the US) program was created in 2010 with the goal to develop and share screening, diagnosis and linkage to care best practices with hospitals, community health centers and community-based organizations in accordance with guidelines recommended by the CDC, US Preventative Services Task Force (USPSTF), and state and local public health departments. Through September 2018, FOCUS partners have conducted more than 5.1 million HIV tests from 261 participating organizations in 83 cities and counties.
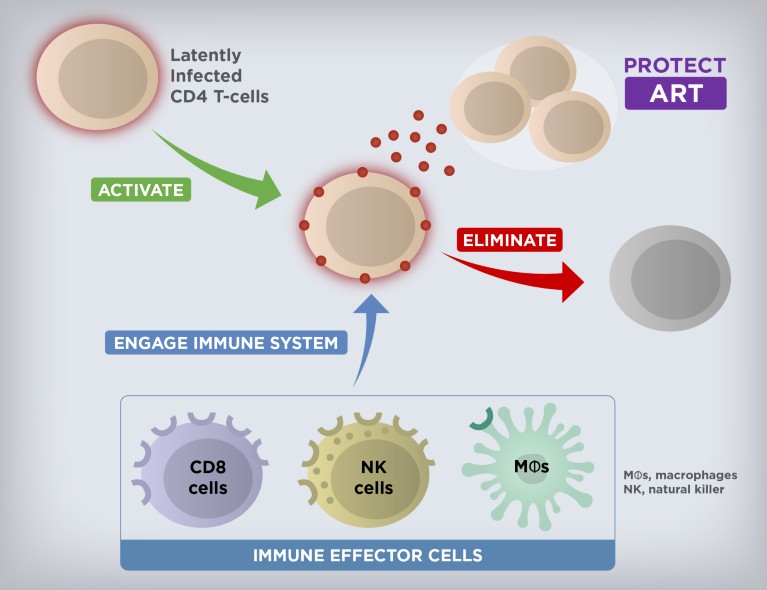
Cure Strategies to Eliminate HIV Reservoirs. Current research is exploring the potential of combination investigational therapies to induce expression of latent HIV and in parallel stimulate and engage host immune functions to specifically target and eliminate the infected cells from the activated reservoir.
Globally, the need is greatest in resource-limited countries with low HIV awareness and lack of education about treatment and prevention options. Since 2006, Gilead has entered into voluntary licensing agreements with generic manufacturers in low- and middle-income countries that grant them the rights to produce and sell high-quality, low-cost generic versions of Gilead medicines. Today, these agreements have expanded access in 116 countries and resulted in an 85 percent reduction in local prices due to manufacturer competition. We also partner with NGOs, governments, and academic institutions to raise awareness, address barriers to treatment access, reduce stigma and deliver frontline services. For example, in Africa, our work with the PEPFAR’s DREAMS initiative provides PrEP medications to young women who are most vulnerable to HIV.
Cure as the Ultimate Goal
While still elusive, cure remains the ultimate long-term goal for Gilead’s HIV research and development efforts. It is well established that the latent HIV reservoir is the main obstacle in achieving cure. 19 While the total size of the HIV reservoir varies among people living with HIV, the latently infected cells are rare (approximately 1 to 100 per million resting CD4+ T cells in the blood). 20 The reservoir is also anatomically heterogeneous and persistent, due to the residence of latent HIV primarily in long-lived memory CD4+ T cells. 21 The quiescent nature of these memory CD4+ T cells makes the reservoir essentially invisible to the immune system and not susceptible to ART. However, if ART is stopped, the virus quickly rebounds, leading to spreading HIV replication and expansion of the reservoir in the vast majority of individuals. 22
A multi-pronged approach will likely be needed to achieve the goal of curing HIV. The discovery and development of latency-reversing agents, immune modulators, and genetically engineered effector antibodies, as well as therapeutic vaccines with a potential for combination therapy to activate and eliminate the persistent reservoir are a current area of focus (Figure 1). Initial results from testing a combination of a TLR7 agonist with a broadly neutralizing antibody or a therapeutic vaccine in non-human primates infected with a chimeric simian-human immunodeficiency virus or simian immunodeficiency virus, respectively, have been a source of cautious optimism 20 , 22 and helped advance several investigational agents into early-stage clinical studies. As we progress further with testing investigational curative regimens, our partnerships and collaborations are more important than ever in this complex effort.
Extraordinary progress has been made in HIV since the epidemic emerged 37 years ago, but significant challenges still remain ahead of us. Gilead is strongly committed to driving the next generation of treatment, prevention, and cure strategy innovations that will continue changing the trajectory of the HIV epidemic by transforming care and improving overall outcomes for all people living with or at risk of HIV.
Barré-Sinoussi F, Ross AL, Delfraissy J. Nature Reviews Microbiology . 2013; 11 (12): 877-883.
Article PubMed Google Scholar
Centers for Disease Control (CDC). Atlas Plus. HIV Diagnoses 2016 https://gis.cdc.gov/grasp/nchhstpatlas/tables.html
CDC. 2016 HIV Surveillance Report, 2016; vol. 28. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf
Global Health Observatory (GHO) Data: HIV/AIDS (2018). http://www.who.int/gho/hiv/en/
Nachega JB, et al. Clinical Infectious Diseases . 2014; 58 (9): 1297–1307.
Bangsberg DR. The Journal of Infectious Diseases . 2008; 197 (3): S272-278.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
Iacob SA & Iacob DG. Ibalizumab. Frontiers in Microbiology . 2017; 8 : 2323.
Barnhart M. Global Health: Science and Practice . 2017; 5 (2): 182–187.
Article Google Scholar
Landovitz RJ, Kofron R, McCauley M. Current Opinion in HIV and AIDS . 2016; 11 (1): 122–128
Carnes CK, Sheehan JH, Aiken C. Current Opinion in HIV and AIDS. 2018; 13 (4): 359–365.
Mendoza P, et al. Nature. 2018; 561 (7724): 479-484
World Health Organization. Policy brief on oral pre-exposure prophylaxis of HIV infection (PrEP). http://apps.who.int/iris/bitstream/handle/10665/197906/WHO_HIV_2015.48_eng.pdf;jsessionid=220ED2F6B4B466872D5082D71FA61E29?sequence=1
Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2017 Update. A Clinical Practice Guideline. Department of Health and Human Services. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
San Francisco Department of Public Health: Population Health Division. HIV Epidemiology Annual Report 2017. https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/AnnualReport2017-Green-20180904-Web.pdf
Kamitani E, et al. AIDS . 2018.
PubMed Google Scholar
Aaron E, et al. AIDS Patient Care STDS. 2018; 32 (1): 16-23.
amfAR. Long-Acting HIV Treatment and Prevention Are Coming: Preparing for Potential Game Changers. https://www.amfar.org/uploadedFiles/_amfarorg/Articles/On_The_Hill/2018/overview.pdf.
Borducchi EN, et al. Nature . 2018; 1.
Google Scholar
Ho YC, et al. Cell. 2013; 155 (3), 540–551
Estes JD, et al. Nature Medicine . 2017; 23 (11): 1271–1276
Borducchi EN, et al. Nature . 2016; 540 : 284-287.
Download references
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Future Directions for HIV Treatment Research
A major goal of NIAID-supported research on HIV treatment today is to develop long-acting therapies that—unlike current antiretrovirals, which require daily dosing—could be taken only once a week, once a month, or even less often. Such long-acting therapies might be easier for some people to stick to than daily pills, and might also be less toxic and more cost effective. The three types of agents under study are long-acting drugs, broadly neutralizing antibodies, and therapeutic vaccines.
Long-Acting Drugs
NIAID-supported scientists aim to develop a new array of drugs for HIV treatment that include longer-acting pills as well as alternative formulations such as injections, patches, and implants. The complexity of developing such products has led NIAID to create a consortium of experts who can facilitate relationships among the many types of researchers needed to translate an idea for a long-acting HIV drug into a workable solution. Called LEAP, for Long-Acting/Extended Release Antiretroviral Resource Program, the consortium includes scientists and clinicians from academia, industry, and government, as well as patient advocates. Read more about LEAP.
NIAID also will investigate the effectiveness of two investigational long-acting HIV drugs, rilpivirine LA and cabotegravir LA, in people for whom adhering to conventional antiretroviral therapy has been a challenge. Another study is planned to test whether the combination of monthly injections of cabotegravir LA and monthly infusions of an NIAID-discovered broadly neutralizing antibody called VRC01LS can keep HIV suppressed in people whose infection was previously controlled by antiretroviral therapy.
Broadly Neutralizing Antibodies
Scientists at the NIAID Vaccine Research Center (VRC) and NIAID-supported scientists at other institutions are developing and testing multiple antibodies for the treatment of HIV. Antibodies are good candidates for treatment because they have few side effects and can be modified to ensure they last a long time in the body, suggesting that dosing could be every other month or even less often. Importantly, the antibodies under investigation can powerfully stop a wide range of HIV strains from infecting human cells in the laboratory and thus are known as broadly neutralizing antibodies, or bNAbs.
In the context of treatment, bNAbs can potentially thwart HIV in three ways:
- By binding directly to the virus, preventing it from entering a cell and accelerating its elimination.
- By binding to an HIV-infected cell, recruiting immune-system components that facilitate cell killing.
- By binding to a key fragment of HIV, forming a complex that may lead to the stimulation of immune cells in a manner similar to a vaccine, thereby preparing the immune system for future encounters with the virus.
Clinical studies have established that giving infusions of certain bNAbs to people living with HIV can suppress the virus, albeit to a limited degree. Further studies have shown that treating people living with HIV with just one bNAb fosters the emergence of HIV strains that are resistant to the antibody. Thus, just as antiretroviral therapy requires a combination of drugs to effectively suppress HIV, it appears that antibody-based therapy will require a combination of either multiple bNAbs or bNAbs and long-acting drugs to suppress the virus. Studies in monkeys infected with a simian version of HIV have already demonstrated that combinations of complementary bNAbs powerfully suppress the virus for an extended period. NIAID is now funding and conducting clinical trials of this strategy for treating HIV in people.
In addition, scientists are engineering changes to known bNAbs to optimize them for HIV treatment and prevention applications. These changes are designed to increase the number of HIV strains an antibody can block, how long the antibody lasts in the body, how powerfully the antibody attaches to the virus, and how efficiently the antibody triggers the immune system to attack both the virus and HIV-infected cells.
Therapeutic HIV Vaccines
Perhaps the ideal treatment for HIV infection would be a therapeutic vaccine. Unlike a vaccine designed to prevent HIV infection, a therapeutic vaccine would be given to people already infected with the virus. Such a vaccine would stimulate the immune system to be ready to control any future emergence of HIV and thereby end the need for further therapy, perhaps save periodic booster shots. Such an approach could lead to sustained viral remission , meaning treatment or vaccination that would result in prolonged undetectable levels of HIV without regular antiretroviral therapy.
The presence of rare people living with HIV who can control the virus naturally either from the time of infection or after halting antiretroviral therapy is evidence that a therapeutic vaccine could theoretically alter the immune system to achieve long-term control of HIV. Nevertheless, attempts to create effective therapeutic HIV vaccines have so far been unsuccessful. To help improve results, NIAID is working to advance the underlying science—in particular, to improve understanding of immune responses that sustainably suppress HIV and to improve the potency of those responses.
Three of the NIAID-funded Martin Delaney Collaboratories are pursuing strategies that involve therapeutic vaccines to achieve long-term control of HIV or reduction of the reservoir of all virus-carrying cells. Read more about the Martin Delaney Collaboratories .
Future Directions for Developing Daily HIV Drugs
At the same time, NIAID continues to support research to develop new drugs with unique mechanisms of action for daily antiretroviral therapy. Such drugs likely would be effective against HIV strains with resistance to other drug types.
For example, basic NIAID-supported research contributed to development of the experimental drug islatravir (also known as EFdA or MK-8591), which belongs to a class of drugs known as nucleoside reverse transcriptase translocation inhibitors, or NRTTIs. NIAID research also contributed to the development of maturation inhibitors, investigational drugs that target the same stage of the HIV lifecycle as protease inhibitors but act by a different mechanism.
Researchers also are attempting to target other parts of the HIV lifecycle. For example, the experimental inhibitor fostemsavir blocks HIV from infecting immune cells by attaching to the gp120 protein on the virus’ surface. Another example is development of capsid assembly inhibitors, which halt construction of the viral capsid, the protein shell that encloses HIV’s genetic material.
For more information on investigational antiretroviral treatments, see the AIDS info Drug Database.


Childhood HIV Vaccination Strategy Shows Promise in Study
- Share to Facebook
- Share to Twitter
- Share to LinkedIn
- Share on Email

Weill Cornell Medicine researchers have developed an experimental vaccine to protect against human immunodeficiency virus (HIV) infection based on " spike" proteins (shown in purple) on the surface of the virus. Credit: Shutterstock
Research at Weill Cornell Medicine suggests that childhood immunization against HIV could one day provide protection before risk of contracting this potentially fatal infection dramatically increases in adolescence.
The study , published Aug. 30 in Science Immunology, demonstrated that a series of six vaccinations containing a modified protein from the surface of HIV particles stimulated initial steps of a potent immune response in young non-human primates. This difficult-to-achieve response represents an important step toward providing full and potentially life-long protection against the virus, the researchers say.
Immunizing young children, rather than adults, makes sense because risk factors for HIV infection rise steeply when adolescents become sexually active, according to senior author Dr. Sallie Permar , the Nancy C. Paduano Professor in Pediatrics and chair of the Department of Pediatrics at Weill Cornell Medicine.
What’s more, evidence suggests that the immune systems of infants and children generally mount more effective responses to the virus than those of adults, said Dr. Permar. “One of the advancements we’ve made is to demonstrate that an HIV vaccine could be delivered on a schedule similar to routine vaccines already given to babies and children.”
Prepping the Immune System Early
HIV predominantly infects immune cells called CD4 T cells, leaving individuals vulnerable to opportunistic diseases. Without lifelong treatment, infection is fatal. In 2022, an estimated 140,000 adolescents between 10 and 19 years old worldwide became infected with the virus—a group that is overrepresented in the number of new infections.

Dr. Sallie Permar
Vaccine researchers are seeking ways to stimulate the immune system to make “broadly neutralizing antibodies” against the virus before a person is exposed to it. These antibodies attack a crucial part of the HIV virus—the protein on its surface that binds to CD4 T cells. In doing so, broadly neutralizing antibodies prevent many strains of HIV from entering the cell and infecting it.
In this study, the researchers started with an experimental vaccine developed previously from spike proteins on the envelope of HIV particles. Study authors Dr. John Moore , a professor of microbiology and immunology, and Dr. Rogier Sanders , an adjunct associate professor of research in microbiology and immunology at Weill Cornell Medicine and a professor at Amsterdam UMC , sought to improve this vaccine by altering the viral protein. They designed these changes to stimulate a specific set of antibody-producing B cells that protect CD4 T cells.
“An effective HIV vaccine needs to engage the right set of B cells in order to generate a broadly protective response,” said first author Dr. Ashley Nelson , an assistant professor of immunology research in pediatrics at Weill Cornell Medicine. “We discovered that introducing certain mutations into the envelope protein could accomplish that in the setting of a naïve immune system.”
Activating the Right B Cells for Protection
The researchers administered the modified vaccine to five young primates in three priming doses, starting less than a week after birth. They followed up with three doses of the vaccine matching the original HIV envelope protein, with the last dose given when the animals reached 78 weeks old, roughly equivalent to four or five years old for a human. As a control, five animals received all six doses of the original envelope protein vaccine.

Dr. Ashley Nelson
“While exposure to the modified protein got the immune response started off in the right direction, booster shots containing the original version of the viral protein were necessary to reach full potential,” Dr. Nelson said.
Three of the five animals who received the modified version of the priming vaccine developed antibodies that appeared to be precursors to the sought-after broadly neutralizing response. Tests suggested these antibodies attacked the site the virus uses to invade CD4 T cells. However, they were not yet fully effective against the same breadth of HIV strains as mature broadly neutralizing antibodies. One of the three animals also showed signs of developing the mature, broadly neutralizing response.
The next step is figuring out how to reliably elicit a full-on broadly neutralizing response, Dr. Nelson said. “We still need to identify the right combination of viral proteins to get us further down that path, starting from the earliest stages in life when multi-dose vaccines are commonly given.”
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, please see the profile for Dr. Sallie Permar .
This work was supported in part by National Institutes of Health (NIH) grant P01 AI117915, Office of Research Infrastructure Program/OD grant P51 OD011107 and by the National Institute of Allergy and Infectious Diseases of the NIH grant P01 AI110657.
Related News
- Computational Approach Yields Novel Cancer Targets
- Analysis Could Guide the Future of Telehealth Policies
- Dr. Margaret McNairy Named NAM Emerging Leader in Health and Medicine
Back to News
Weill Cornell Medicine Office of External Affairs New York, NY --> Phone: (646) 962-9476
New study highlights potential of childhood immunization against HIV
- Download PDF Copy
Research at Weill Cornell Medicine suggests that childhood immunization against HIV could one day provide protection before risk of contracting this potentially fatal infection dramatically increases in adolescence.
The study, published Aug. 30 in Science Immunology, demonstrated that a series of six vaccinations containing a modified protein from the surface of HIV particles stimulated initial steps of a potent immune response in young non-human primates. This difficult-to-achieve response represents an important step toward providing full and potentially life-long protection against the virus, the researchers say.
Immunizing young children, rather than adults, makes sense because risk factors for HIV infection rise steeply when adolescents become sexually active, according to senior author Dr. Sallie Permar, the Nancy C. Paduano Professor in Pediatrics and chair of the Department of Pediatrics at Weill Cornell Medicine.
What's more, evidence suggests that the immune systems of infants and children generally mount more effective responses to the virus than those of adults. One of the advancements we've made is to demonstrate that an HIV vaccine could be delivered on a schedule similar to routine vaccines already given to babies and children." Dr. Sallie Permar, the Nancy C. Paduano Professor in Pediatrics and chair of the Department of Pediatrics at Weill Cornell Medicine
Prepping the immune system early
HIV predominantly infects immune cells called CD4 T cells , leaving individuals vulnerable to opportunistic diseases. Without lifelong treatment, infection is fatal. In 2022, an estimated 140,000 adolescents between 10 and 19 years old worldwide became infected with the virus—a group that is overrepresented in the number of new infections.
Vaccine researchers are seeking ways to stimulate the immune system to make "broadly neutralizing antibodies " against the virus before a person is exposed to it. These antibodies attack a crucial part of the HIV virus—the protein on its surface that binds to CD4 T cells. In doing so, broadly neutralizing antibodies prevent many strains of HIV from entering the cell and infecting it.
In this study, the researchers started with an experimental vaccine developed previously from spike proteins on the envelope of HIV particles. Study authors Dr. John Moore, a professor of microbiology and immunology, and Dr. Rogier Sanders, an adjunct associate professor of research in microbiology and immunology at Weill Cornell Medicine and a professor at Amsterdam UMC, sought to improve this vaccine by altering the viral protein. They designed these changes to stimulate a specific set of antibody-producing B cells that protect CD4 T cells.
Related Stories
- Can odors help fight infection? Nematode research suggests so
- Garlic’s antioxidant and nitric oxide boosting effects may help lower blood pressure
- Untreated hypertension increases Alzheimer’s risk, research shows
"An effective HIV vaccine needs to engage the right set of B cells in order to generate a broadly protective response," said first author Dr. Ashley Nelson, an assistant professor of immunology research in pediatrics at Weill Cornell Medicine. "We discovered that introducing certain mutations into the envelope protein could accomplish that in the setting of a naïve immune system."
Activating the right B cells for protection
The researchers administered the modified vaccine to five young primates in three priming doses, starting less than a week after birth. They followed up with three doses of the vaccine matching the original HIV envelope protein, with the last dose given when the animals reached 78 weeks old, roughly equivalent to four or five years old for a human. As a control, five animals received all six doses of the original envelope protein vaccine.
"While exposure to the modified protein got the immune response started off in the right direction, booster shots containing the original version of the viral protein were necessary to reach full potential," Dr. Nelson said.
Three of the five animals who received the modified version of the priming vaccine developed antibodies that appeared to be precursors to the sought-after broadly neutralizing response. Tests suggested these antibodies attacked the site the virus uses to invade CD4 T cells. However, they were not yet fully effective against the same breadth of HIV strains as mature broadly neutralizing antibodies. One of the three animals also showed signs of developing the mature, broadly neutralizing response.
The next step is figuring out how to reliably elicit a full-on broadly neutralizing response, Dr. Nelson said. "We still need to identify the right combination of viral proteins to get us further down that path, starting from the earliest stages in life when multi-dose vaccines are commonly given."
Weill Cornell Medicine
Nelson, A. N., et al. (2024) Immunization with germ line–targeting SOSIP trimers elicits broadly neutralizing antibody precursors in infant macaques . Science Immunology . doi.org/10.1126/sciimmunol.adm7097 .
Posted in: Child Health News | Medical Research News | Disease/Infection News
Tags: Adolescents , Allergy , Antibodies , Antibody , CD4 , Cell , Children , HIV , Immune Response , Immune System , Immunization , Immunology , Infectious Diseases , Medicine , Microbiology , Pediatrics , Protein , Research , Vaccine , Virus
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

How can microdialysis benefit drug development
Ilona Vuist
In this interview, discover how Charles River uses the power of microdialysis for drug development as well as CNS therapeutics.

Global and Local Efforts to Take Action Against Hepatitis
Lindsey Hiebert and James Amugsi
In this interview, we explore global and local efforts to combat viral hepatitis with Lindsey Hiebert, Deputy Director of the Coalition for Global Hepatitis Elimination (CGHE), and James Amugsi, a Mandela Washington Fellow and Physician Assistant at Sandema Hospital in Ghana. Together, they provide valuable insights into the challenges, successes, and the importance of partnerships in the fight against hepatitis.

Addressing Important Cardiac Biology Questions with Shotgun Top-Down Proteomics
In this interview conducted at Pittcon 2024, we spoke to Professor John Yates about capturing cardiomyocyte cell-to-cell heterogeneity via shotgun top-down proteomics.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback

- What Are HIV and AIDS?
- How Is HIV Transmitted?
- Who Is at Risk for HIV?
- Symptoms of HIV
- U.S. Statistics
- Impact on Racial and Ethnic Minorities
- Global Statistics
- HIV and AIDS Timeline
- In Memoriam
- Supporting Someone Living with HIV
- Standing Up to Stigma
- Getting Involved
- HIV Treatment as Prevention
- Pre-exposure Prophylaxis (PrEP)
- Post-exposure Prophylaxis (PEP)
- Preventing Sexual Transmission of HIV
- Alcohol and HIV Risk
- Substance Use and HIV Risk
- Preventing Perinatal Transmission of HIV
- HIV Vaccines
- Long-acting HIV Prevention Tools
- Microbicides
- Who Should Get Tested?
- HIV Testing Locations
- HIV Testing Overview
- Understanding Your HIV Test Results
- Living with HIV
- Talking About Your HIV Status
- Locate an HIV Care Provider
- Types of Providers
- Take Charge of Your Care
- What to Expect at Your First HIV Care Visit
- Making Care Work for You
- Seeing Your Health Care Provider
- HIV Lab Tests and Results
- Returning to Care
- HIV Treatment Overview
- Viral Suppression and Undetectable Viral Load
- Taking Your HIV Medicine as Prescribed
- Tips on Taking Your HIV Medicine as Prescribed
- Paying for HIV Care and Treatment
- Other Health Issues of Special Concern for People Living with HIV
- Alcohol and Drug Use
- Coronavirus (COVID-19) and People with HIV
- Hepatitis B & C
- Vaccines and People with HIV
- Flu and People with HIV
- Mental Health
- Mpox and People with HIV
- Opportunistic Infections
- Sexually Transmitted Infections
- Syphilis and People with HIV
- HIV and Women's Health Issues
- Aging with HIV
- Emergencies and Disasters and HIV
- Employment and Health
- Exercise and Physical Activity
- Nutrition and People with HIV
- Housing and Health
- Traveling Outside the U.S.
- Civil Rights
- Workplace Rights
- Limits on Confidentiality
- National HIV/AIDS Strategy (2022-2025)
- Implementing the National HIV/AIDS Strategy
- Prior National HIV/AIDS Strategies (2010-2021)
- Key Strategies
- Priority Jurisdictions
- HHS Agencies Involved
- Learn More About EHE
- Ready, Set, PrEP
- Ready, Set, PrEP Pharmacies
- AHEAD: America’s HIV Epidemic Analysis Dashboard
- HIV Prevention Activities
- HIV Testing Activities
- HIV Care and Treatment Activities
HIV Research Activities
- Activities Combating HIV Stigma and Discrimination
- The Affordable Care Act and HIV/AIDS
- HIV Care Continuum
- Syringe Services Programs
- Finding Federal Funding for HIV Programs
- Fund Activities
- The Fund in Action
- About PACHA
- Members & Staff
- Subcommittees
- Prior PACHA Meetings and Recommendations
- I Am a Work of Art Campaign
- Awareness Campaigns
- Global HIV/AIDS Overview
- U.S. Government Global HIV/AIDS Activities
- U.S. Government Global-Domestic Bidirectional HIV Work
- Global HIV/AIDS Organizations
- National Black HIV/AIDS Awareness Day February 7
- HIV Is Not A Crime Awareness Day February 28
- National Women and Girls HIV/AIDS Awareness Day March 10
- National Native HIV/AIDS Awareness Day March 20
- National Youth HIV & AIDS Awareness Day April 10
- HIV Vaccine Awareness Day May 18
- National Asian & Pacific Islander HIV/AIDS Awareness Day May 19
- HIV Long-Term Survivors Awareness Day June 5
- National HIV Testing Day June 27
- Zero HIV Stigma July 21
- Southern HIV/AIDS Awareness Day August 20
- National Faith HIV/AIDS Awareness Day August 25
- National African Immigrants and Refugee HIV/AIDS and Hepatitis Awareness Day September 9
- National HIV/AIDS and Aging Awareness Day September 18
- National Gay Men's HIV/AIDS Awareness Day September 27
- National Latinx AIDS Awareness Day October 15
- World AIDS Day December 1
- Event Planning Guide
- U.S. Conference on HIV/AIDS (USCHA)
- National Ryan White Conference on HIV Care & Treatment
- AIDS 2020 (23rd International AIDS Conference Virtual)
Want to stay abreast of changes in prevention, care, treatment or research or other public health arenas that affect our collective response to the HIV epidemic? Or are you new to this field?
HIV.gov curates learning opportunities for you, and the people you serve and collaborate with.
Stay up to date with the webinars, Twitter chats, conferences and more in this section.
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share on Email
Supporting Research to Effectively Prevent, Diagnose, and Treat HIV
In the three decades since the first cases of AIDS were reported, Federal investments in basic, biomedical, behavioral, and social science research have led to numerous HIV prevention interventions and life-saving treatments. Several federal agencies conduct or support HIV research activities.
Leading the Way in HIV Research
The National Institutes of Health (NIH), a part of the U.S. Department of Health and Human Services (HHS), is the Nation’s primary medical research agency, making important discoveries that improve health and save lives. NIH conducts and supports a comprehensive program of basic, behavioral, clinical, and translational research on HIV/AIDS and its associated coinfections, comorbidities, and other complications. Since HIV crosses nearly every area of medicine and scientific investigation, the response to the HIV pandemic requires a multi-Institute, multidisciplinary, global research program. At NIH, this research is coordinated by the Office of AIDS Research (OAR) and carried out by nearly all the NIH Institutes and Centers, in both at NIH and at NIH-funded institutions worldwide. The NIH HIV/AIDS Research Program represents the world’s largest public investment in AIDS research.
Within NIH, the National Institute of Allergy and Infectious Diseases (NIAID) manages the largest portfolio of HIV/AIDS research activities. NIAID-supported investigators have made groundbreaking scientific discoveries that have led to significant progress in the fight against HIV/AIDS. Among these discoveries, NIAID-supported research has demonstrated that people living with HIV who take antiretroviral medications daily as prescribed and who achieve and maintain an undetectable viral load have effectively no risk of sexually transmitting the virus to an HIV-negative partner .
The NIAID-supported HPTN 052 study , which involved more than 1,600 heterosexual couples over 10 years, found that starting and sustaining treatment for HIV infection early, when the immune system is relatively healthy, essentially eliminated the transmission of HIV. No HIV transmission was observed when antiretroviral therapy consistently, durably suppressed the virus in the partner living with HIV. While some transmission events did occur in the study, new infections resulted when the partner living with HIV was not fully virally suppressed due to either having just started antiretroviral therapy, or for whom treatment no longer was working and the virus was replicating. The study also showed that early treatment initiation improved health outcomes for people living with HIV.
The HPTN 052 results, along with those of another NIAID-funded trial (the START study ), which showed that those living with HIV who received early treatment significantly reduced their risk of illness and death, helped influence the World Health Organization in 2015 to recommend that everyone living with HIV should begin treatment upon diagnosis.
Seeking a Cure for HIV
NIAID also invests in basic and clinical research with the goal of developing a safe, affordable and scalable cure for HIV and AIDS. NIAID’s research efforts include studies to identify the precise locations where HIV hides in the body (known as viral reservoirs), determine how those reservoirs are established and maintained, and develop strategies to minimize or deplete them. An HIV cure in the classic sense, meaning it removes all HIV from the body, would require eradication of viral reservoirs. Treatment-free remission, also known as a functional cure, would not eradicate reservoirs but would allow a person living with HIV to control the virus without daily medication.
Making Progress Toward an HIV Vaccine
Historically, vaccination has been the best method for protecting people from infectious diseases. While many HIV prevention techniques are available, the development of a safe and effective HIV vaccine remains key to realizing a durable end to the HIV/AIDS pandemic. NIAID and its global partners are pursuing numerous research strategies to develop next-generation vaccine candidates.
Developing Safe and Effective HIV Treatments
One of NIAID’s greatest success stories is that its research led to the development of numerous antiretroviral drugs to treat HIV/AIDS, turning what was once a uniformly fatal disease into a manageable chronic condition for many. NIAID is working to find new and more effective therapeutic products, drug classes, and combinations as well as safe and effective treatments for related co-infections and complications.
Research to Prevent HIV Infection and Transmission
NIAID also conducts and supports research to develop and improve cutting-edge tools and techniques that can work to prevent HIV in diverse populations around the world.
The Centers for Disease Control and Prevention (CDC) also provides national leadership for HIV prevention research , including the development of biomedical and behavioral interventions to prevent HIV transmission and reduce disease progression in the United States and internationally. CDC’s research efforts include identifying scientifically proven, cost-effective, and scalable interventions and prevention strategies to be implemented as part of a high-impact prevention approach for maximal impact on the HIV epidemic.
Advancing the National HIV Priorities through Research
The National HIV/AIDS Strategy: Updated to 2020 calls for numerous ongoing research efforts, including the prioritization and promotion of research to fill in gaps in prevention science among the highest risk populations and communities; the promotion and prioritization of research to fill in gaps in knowledge along the HIV care continuum; the scaling up of effective, evidence-based programs that address social determinants of health; support for research to better understand the scope of the intersection of HIV and violence against women and girls, as well as the development of effective interventions; and the strengthening of the timely availability and use of data. Across the Federal government, agencies and programs are engaged in these efforts.
Scroll down to read about the HIV research activities of individual Federal agencies and offices.
Related HIV.gov Blogs
Cdc announces availability of $7 million to accelerate prep uptake in ehe jurisdictions, icymi: research roundup from aids 2024, cdc awards hiv prevention and surveillance funding to health departments.
- Clinical Trials
- Cure Research HIV Cure Research
- Vaccine HIV Vaccine
- HIV Vaccine Awareness Day
- AHRQ Agency for Healthcare Research & Quality
- CDC Centers for Disease Control & Prevention
- DoD Department of Defense
- FDA Food & Drug Administration
- NIH National Institutes of Health
Federal Agencies' Research Activities

The Agency for Healthcare Research and Quality (AHRQ) produces evidence to make health care safer, higher quality, more accessible, equitable, and affordable, and works within the U.S. Department of Health and Human Services and with other partners to make sure that the evidence is understood and used to achieve the goals of better care, smarter spending of health care dollars, and healthier people.
AHRQ produces numerous research reports on public health, including the Congressionally-mandated National Healthcare Quality Report and the National Healthcare Disparities Report, which include sections on HIV/AIDS and its effective treatment.
Learn more about AHRQ.

The Centers for Disease Control and Prevention (CDC), part of the U.S. Department of Health and Human Services, works 24/7 to protect America from health, safety, and security threats. CDC provides leadership in helping control the HIV/AIDS epidemic by working with community, state, national, and international partners in surveillance, research, and prevention and evaluation activities, as well as working to improve treatment and support for people living with HIV.
Within CDC, the National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention leads its HIV/AIDS activities. The Center’s Division of HIV/AIDS Prevention (DHAP) is charged with the mission of preventing HIV infection and reducing the incidence of HIV-related illness and death in the U.S.
CDC provides national leadership for HIV prevention research, including the development and evaluation of HIV biomedical and behavioral interventions to prevent HIV transmission and reduce HIV disease progression in the United States and internationally. CDC’s research efforts also include identifying those scientifically proven, cost-effective, and scalable interventions and prevention strategies to be implemented as part of a high-impact prevention approach for maximal impact on the HIV epidemic.
Learn more about CDC’s HIV research efforts.
View CDC’s activities in the National HIV/AIDS Strategy Federal Action Plan.

The mission of the U.S. Department of Defense (DoD) is to provide the military forces needed to deter war and to protect the security of our country. Within the DoD, the U.S. Military HIV Research Program (MHRP) is at the forefront of the battle against HIV to protect U.S. troops and reduce the global impact of the disease. Since its inception in 1986, MHRP has become a world leader in HIV vaccine research, threat assessment, epidemiology, HIV diagnostics, and cure research. The program works in six sites in Africa and Asia. While its primary focus is developing a globally effective vaccine, the program also provides prevention, care, and treatment in each of the communities in which research is conducted through the President’s Emergency Plan for AIDS Relief (PEPFAR) . MHRP is centered at the Walter Reed Army Institute of Research (WRAIR), a command within the U.S. Army Medical Research and Materiel Command.
Learn more about the MHRP.
View DoD’s activities in the National HIV/AIDS Strategy Federal Action Plan.

The Food and Drug Administration (FDA ), an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products, and medical devices. FDA is also responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off radiation, and for regulating tobacco products. FDA's broad based, multi-disciplinary research programs have played a significant role in the development of vaccines, therapeutic agents, and test kits for use in HIV/AIDS and HIV-related conditions. This research includes work on HIV infection and vaccine models for its prevention, and studies of the immune response to HIV, as well as conducting, planning, or consulting on epidemiology studies of the role devices or radiation play in the transmission, prevention, detection, or treatment of HIV infection and closely associated conditions.
FDA also plays a role in overseeing cure research for HIV, ensuring that risks are out of proportion to the potential benefits, and that patients who are willing to enter into HIV clinical trials are aware of the potential risks that may be associated with different approaches, particularly for patients who are on stable, effective drug therapies.
Learn more about FDA’s role in HIV/AIDS.
Agency for Healthcare Research and Quality
Centers for disease control and prevention, department of defense, food and drug administration.

The National Institutes of Health (NIH), part of the U.S. Department of Health and Human Services, is the nation’s medical research agency—making important medical discoveries that improve health and save lives. NIH-funded scientists investigate ways to prevent disease and conduct research on both common and rare diseases to discover their causes, develop effective treatments, and find cures. NIH represents the largest and most significant public investment in HIV/AIDS research in the world. Almost all of the 27 NIH Institutes and Centers (ICs) conduct and support basic, clinical, behavioral, social science, and translational research that addresses the prevention and treatment of HIV disease and its associated coinfections, comorbidities, and other complications.
NIH’s Office of AIDS Research (OAR) coordinates the scientific, budgetary, legislative, and policy elements of the trans-NIH HIV/AIDS-related research programs. OAR, a component of the Division of Program Coordination, Planning, and Strategic Initiatives in the Office of the NIH Director, supports the development of up-to-date HHS guidelines for the treatment of HIV infection, and the prevention and treatment of HIV-associated opportunistic infections. The guidelines are developed and regularly updated by working groups of HIV experts from across the country, including physicians, other health care clinical providers, pharmacists, researchers, and HIV treatment advocates. These clinical guidelines outline the current science and recommendations for treatment of HIV disease (e.g., antiretroviral therapy, treatment, and prophylaxis for opportunistic infections) as well as guidelines for conducting HIV testing and counseling.
Within NIH, the National Institute of Allergy and Infectious Diseases (NIAID) leads research to understand, treat, and prevent infectious, immunologic, and allergic diseases, including HIV/AIDS. Through laboratories and clinics on the NIH campus in Bethesda, Maryland, and a vast network of supported research at universities, medical centers, and clinical trial sites around the globe, NIAID is working to better understand HIV and how it causes disease, find new tools to prevent HIV infection including a preventive vaccine, develop new and more effective treatments for people with HIV, and hopefully, find a cure.
In addition to NIAID, NIH is made up of 26 other Institutes and Centers . Each has its own specific research agenda, often focusing on particular diseases or body systems, and most address HIV/AIDS in some way, according to their particular area of expertise.
Learn more about NIH’s HIV/AIDS Research Program.
Learn more about NIH’s role in the National HIV/AIDS Strategy Federal Action Plan.
National Institutes of Health
Our websites may use cookies to personalize and enhance your experience. By continuing without changing your cookie settings, you agree to this collection. For more information, please see our University Websites Privacy Notice .
UConn Today
September 3, 2024 | Anna Zarra Aldrich, College of Agriculture, Health and Natural Resources
New Study Reveals Relationship Between HIV Risk Factors for LGBTQ+ Youth
A new study has uncovered empirical evidence that shows the importance of taking a holistic approach to addressing HIV risk factors
Photo by Betzy Arosemena for Unspalsh)
A new study has uncovered empirical evidence that shows what researchers have long suspected about HIV risk – that having multiple risk factors is much worse than having only one.
Pablo Kokay Valente, assistant professor of allied health sciences in the College of Agriculture, Health and Natural Resources ( CAHNR ) led this study in collaboration with Ryan Watson, associate professor, and Lisa Eaton, professor, both in the Department of Human Development and Family Sciences. The study was recently published in the American Journal of Public Health.

For a long time, researchers looked at each of these factors independently. But recently there has been a push to consider their interactions.
Most papers looking at these factors have demonstrated a linear relationship. What this means is that if you have one factor at play – say, for example, depression – it is twice as bad to have two factors, like depression and alcohol use.
This new paper demonstrates an exponential relationship instead. This is something that scientists have theorized for years without much empirical evidence to support the hypothesis until now.
“Most studies haven’t been able to demonstrate this kind of synergistic relationship between the syndemic factors,” Valente says. “And that’s what we did. We’ve shown that having two factors is much worse than having one and having three factors is much, much worse than having two factors.”
The researchers used data from a survey of LGBTQ+ youth. LGBTQ+ people have historically and continue to be one group that is at a greater risk of contracting HIV.
“A major strength of this study was the use of our national sample of LGBTQ+ youth, many of whom reported intersections of multiple marginalized social positions,” Watson says. “Data collected with so many young LGBTQ+ youth give us a unique view into the complexities and nuances of the lived experiences of today’s LGBTQ+ teens.”
One unexpected finding is that the more factors an individual had, the more likely they were to be exposed to PrEP – a medication that can prevent the contraction of HIV even if you come into contact with the virus – and get information about the drug and its benefits.
“People who are exposed to these factors in combination, they have much more risk and they are probably more connected and more aware of what’s out there in terms of PrEP,” Valente says.
This understanding changes the way researchers think about interventions for people at risk of contracting HIV.
“Better understanding syndemic conditions in one of the most vulnerable youth populations — sexual and gender diverse adolescents — has been much needed, and this study contributes to the growing body of research by using a large national sample of LGBTQ+ youth,” Watson says.
If the linear model were accurate, it would not matter which HIV risk factor interventions were addressed since they all have, in theory, the same impact. But an exponential relationship demonstrates the need for interventions that tackle multiple risk factors at once to provide a substantial benefit.
“If they are linear, the implication is that whatever you address, there is some benefit to that,” Valente says. “Showing that it’s synergistic, it calls for interventions that address more than one of these things. Addressing two of these factors would have more of an impact than addressing things individually.”
For example, therapy-based interventions that address stigma surrounding HIV may also reduce substance use among participants, since that is a common coping strategy for stigmatization, and improve their overall mental health.
“They’re all deeply related,” Valente says. “So, I think dismantling several of them at a time will be very important.”
Valente is continuing to use the methods that uncovered the exponential relationship among LGBTQ+ people with a dataset of hospitals that provide care for people living with HIV.
This work relates to CAHNR’s Strategic Vision area focused on Enhancing Health and Well-Being Locally, Nationally, and Globally and Promoting Diversity, Equity, Inclusion, and Justice.
Follow UConn CAHNR on social media
Recent Articles

September 6, 2024
Professor Folta to Co-Lead National Academy of Sciences Committee
Read the article

‘Mouth Taping’ Not the Answer for Better Sleep

September 5, 2024
The School of Nursing’s Largest First-Year Class
- Academic Overview
- BSN Program
- MSN Program
- DNP Program
- PhD Program
- Post-Graduate Program
- UT Health Services
- Faculty Practice
- Nursing Research
- Educational Innovation
- Diversity at Cizik
- About Cizik Nursing
- Prospective Students
- Current Students
- Cizik Careers
- CSON Intranet
- INSIDE THE UNIVERSITY
- Our Faculty
- Cizik Nursing Intranet
- Search --> Query
- BSN Program Overview
- MSN Program Overview
- Family Nurse Practitioner
- Nursing Leadership
- DNP Program Overview
- BSN-DNP Nurse Anesthesia
- Adult/Gerontology Acute Care Nurse Practitioner
- Adult/Gerontology Primary Care Nurse Practitioner
- Psychiatric/Mental Health Nurse Practitioner
- Nurse Anesthesia
- Nurse Executive
- Nurse Practitioner/CNS
- Nursing Informatics
- PhD Program Overview
- PhD (Post-master's Entry)
- Post-Graduate Program Overview
- Emergency/Trauma Care
- Nursing Education
Barr advances research on HIV and lactation support
Written by: Merve Erten | Updated: September 05, 2024

After decades of being told that parents with HIV are not able to breast/chestfeed their infants, research shows that highly active antiretroviral HIV therapy reduces the chances of HIV transmission to the newborn.
To help spread the word, Cizik School of Nursing at UTHealth Houston Assistant Professor Emily Barr, PhD, CPNP-PC, CNM, ACRN, FACNM, FAAN, will use a $5,000 Dean’s Research Award to further her research on improving HIV knowledge and training for lactation consultants using telehealth to support people living with HIV in their infant feeding decisions.
Typically, parents with HIV in the United States and other higher income countries have been advised to use replacement feeding, like formula, due to the risk of transmitting the HIV virus to their babies through human milk. However, research has shown that the risk of transmission has significantly decreased, falling from 30-50% to less than 1% when HIV is fully suppressed with antiretroviral medications. This led to a significant policy change in January 2023 when the U.S. Department of Health and Human Services updated guidelines, recommending women and other parents with HIV who are virologically suppressed be offered a choice to breast/chestfeed their infants.
Despite this policy change, awareness remains limited, leading to inconsistent practices among health care providers, noted Dr. Barr, an expert in HIV care with over 30 years of experience in the field.
“Every situation is still unique, and a lot of people don’t know about the new guidelines,” she said. “You’d have a woman with HIV show up to deliver a baby on a unit, and then she wants to breastfeed, but the nurses on the unit don’t know that it’s safe. They say, ‘No, you can’t breastfeed because you have HIV.’”
Recognizing this gap, Dr. Barr surveyed 203 lactation consultants in the United States and Canada to assess their knowledge of HIV, particularly in the context of breast/chestfeeding, and their experiences with telehealth – a practice that has gained popularity since the COVID-19 pandemic.
“Lactation consultants offer critical specialized support, and including their telehealth experience was important,” she said. “Consultants could be based centrally, but provide what they call ‘tele-lactation’ to support to people with HIV who are breastfeeding, regardless of their location."
Preliminary findings from the study revealed that while lactation consultants generally have a good understanding of HIV, their knowledge of the nuances of HIV and breastfeeding is relatively low. Furthermore, the study highlighted a moderate level of HIV stigma among lactation consultants, reflecting the need for targeted education and training.
The Dean’s Research Award will support the completion of the quantitative analysis of the survey data, with plans to publish the findings.
“Our goal is training the lactation consultants to be able to increase their knowledge about how to specifically support people who are living with HIV and breastfeeding or chestfeeding,” she said. “We will also address ingrained stigma, with the ultimate goal of testing its effectiveness through a larger grant.”
Co-investigators on the study, “Tele-lactation for breast/chestfeeding parents with HIV: An examination of HIV-knowledge, attitudes, experiences, and HIV stigma in certified lactation consultants in the United States and Canada,” are Assistant Professor Rebecca Tsusaki, PhD, WHNP-BC, IBCLC MPH, of Cizik School of Nursing; UTHealth Houston School of Public Health statistician Hulin Wu, PhD; Jennifer McKinney, MD, of Baylor College of Medicine; Lisa Abuogi, MD, of University of Colorado; and Elizabeth Lowenthal, MD, of Children’s Hospital of Philadelphia.
Merve Erten
Read more about
In this story
Emily a barr, phd, rn, cpnp-pc, cnm, acrn, facnm, faan.

Student Resources
- TMC Library
- Directions and Parking
Web Resources
- Faculty Directory
- Cizik Intranet
- Web File Viewing
Quick Links
- Emergency Information
- How to Report Sexual Misconduct
- Site Policies
- State of Texas
HIV research grants for students underrepresented in medicine
Medical Student Education Sep 06, 2024
Seeking medical students from communities that are underrepresented in medicine in the US who are considering careers in HIV prevention and/or vaccine research. Application Deadline: Monday, December 2 Want to learn more? Join us for a RAMP Informational Webinar
Wednesday, September 18 2:00 pm – 3:00 pm PT (5:00 pm – 6:00 pm ET)
The HIV Vaccine Trials Network, in collaboration with the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, is investing in a young generation of HIV prevention researchers by providing medical students from communities that are underrepresented in medicine in the US with opportunities to conduct independent research while receiving mentoring, project and salary funding, training, and professional development opportunities.
The Request for Applications can be found on October 1.
Grant recipients will be mentored by HVTN-affiliated investigators while conducting research projects in areas of basic, clinical, behavioral, and social science. Projects will be:
Short-Term Projects: 8-10 weeks
Timed with a summer break or fourth-year research elective Travel to an HIV Vaccine Clinical Research Site in the US or abroad Attend and present at an HVTN Conference Maximum award of $20,000
Project Options
During the application process, scholars will have the opportunity to rank all of the research projects according to their interests. Projects for the 2025-2026 cycle will be posted on October 1.
To see the projects of our current and former scholars , check out their profiles.
Eligibility Criteria
Medical students who self-identify as being from communities that are underrepresented in medicine in the US Attending US MD, DO programs, or international medical schools that are US certification eligible (ECFMG) In good academic standing US citizen or US permanent resident or DACA status Interested in pursuing a future career in HIV research
Application Materials
To apply, visit http://www.hvtn.org/ramp . The Request For Applications (RFA) and application materials will be available on October 1. Carefully read the Request for Applications before applying. Applications are due online by 5:00 PM PT on Monday, December 2.
For More Information All correspondence concerning the program should be addressed to:
Linda Oseso, MPH RAMP Program Manager HIV Vaccine Trials Network
Fred Hutchinson Cancer Center 1100 Fairview Ave. N., Mail Stop M2-B500 Seattle, WA 98109 206-667-2175 [email protected]
Medical Student Education
The Medical Student Education team includes student affairs, curricular affairs and student support professionals across the state who support medical students at every step of their journey.
Suggested for you

IMAGES
VIDEO
COMMENTS
Advances in HIV/AIDS Research. HIV virions budding and releasing from an infected cell. NIAID, NIH. For an update on what medical science is doing to fight the global HIV/AIDS pandemic, read a Parade article by NIH Director Francis S. Collins and NIAID Director Anthony S. Fauci, AIDS in 2010: How We're Living with HIV.
Research. CDC provides national leadership for HIV prevention research, including the development and evaluation of HIV biomedical and behavioral interventions to prevent HIV transmission and reduce HIV disease progression in the United States and internationally. CDC's research efforts also include identifying those scientifically proven ...
This is how the world finally ends the HIV/AIDS pandemic
HIV and AIDS
Approximately 1.2 million people in the United States are currently living with HIV, and an estimated 14% are infected, yet unaware of their status (Office of Infectious Disease and HIV/AIDS Policy, 2020).HIV and AIDS continue to have a disproportionate impact on certain populations, including youth—gay, bisexual, and other men who have sex with men (MSM)—racial and ethnic minorities ...
The edited cells underwent affinity maturation in vivo, improving the potency of HIV-1 and SARS-CoV-2 neutralizing antibodies without loss of bioavailability. Affinity maturation of edited cells ...
An effective and scalable cure strategy is a top priority for the HIV research field; this Review discusses recent advances, knowledge gaps, and priority research areas for the next 5 years.
The Lancet HIV Home Page
Welcome to OAR. The Office of AIDS Research (OAR) coordinates HIV/AIDS research across the National Institutes of Health (NIH). The NIH provides the largest public investment in HIV/AIDS research globally. As HIV crosses nearly every area of medicine and scientific investigation, the response to the HIV pandemic requires a multi-Institute ...
The global HIV response has made tremendous progress but is entering a new phase with additional challenges. Scientific innovations have led to multiple safe, effective, and durable options for treatment and prevention, and long-acting formulations for 2-monthly and 6-monthly dosing are becoming available with even longer dosing intervals possible on the horizon. The scientific agenda for HIV ...
Researchers a step closer to a cure for HIV. A new study shows virus-like particle can effectively 'shock and kill' latent HIV reservoir in those living with chronic HIV. By 2030, the World Health ...
More than 3,600 HIV and infectious disease researchers from 73 countries gathered in Denver and virtually from March 3-6 this year for CROI, an annual scientific meeting on the latest research that can help accelerate global progress in the response to HIV and other infectious diseases, including STIs and viral hepatitis.
Investing in research to find a cure for HIV is focused on two broad aims: sustained viral remission and, in the longer term, viral eradication. Viral latency and sanctuaries. Latent HIV reservoirs—small amounts of HIV that persist in people taking ART—present a significant challenge to finding a cure for HIV. Latent reservoirs remain in ...
After decades of failures, researchers have renewed ...
NIH's Dr. Carl Dieffenbach Discusses Highlights of HIV Cure, Treatment and Prevention Research from CROI 2022. 02-17-2022 In an HIV.gov video conversation yesterday, NIH's Dr. Carl Dieffenbach discussed some of the pivotal HIV research advances presented this week at the 2022….
HIVinfo | Information on HIV/AIDS Treatment, Prevention and ...
08-15-2024 A brief roundup of key topics of HIV.gov s coverage of AIDS 2024, including HIV prevention, cure research, and more. Topics AIDS 2024 International AIDS Conference 2024 NIAID National Institute of Allergy & Infectious Diseases NIH National Institutes of Health Prevention Research.
Thanks to innovative research, scientists learned how the HIV virus that causes AIDS replicates and how the immune system responds to the virus. Today, many people with HIV take just one pill a day to suppress the virus, and treatment is continuing to evolve. In this video, Dr. Stacey Rizza, Mayo Clinic infectious disease physician and HIV ...
HIV: Progress and future challenges in treatment, ...
A major goal of NIAID-supported research on HIV treatment today is to develop long-acting therapies that—unlike current antiretrovirals, which require daily dosing—could be taken only once a week, once a month, or even less often. Such long-acting therapies might be easier for some people to stick to than daily pills, and might also be less ...
HIV/AIDS research. Scanning electron micrograph of HIV-1, colored green, budding from a cultured lymphocyte. Diagram of HIV. HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
"An effective HIV vaccine needs to engage the right set of B cells in order to generate a broadly protective response," said first author Dr. Ashley Nelson, an assistant professor of immunology research in pediatrics at Weill Cornell Medicine. "We discovered that introducing certain mutations into the envelope protein could accomplish ...
Research at Weill Cornell Medicine suggests that childhood immunization against HIV could one day provide protection before risk of contracting this potentially fatal infection dramatically ...
NIAID's research efforts include studies to identify the precise locations where HIV hides in the body (known as viral reservoirs), determine how those reservoirs are established and maintained, and develop strategies to minimize or deplete them. An HIV cure in the classic sense, meaning it removes all HIV from the body, would require ...
A new study has uncovered empirical evidence that shows the importance of taking a holistic approach to addressing HIV risk factors. ... and this study contributes to the growing body of research by using a large national sample of LGBTQ+ youth," Watson says.
To help spread the word, Cizik School of Nursing at UTHealth Houston Assistant Professor Emily Barr, PhD, CPNP-PC, CNM, ACRN, FACNM, FAAN, will use a $5,000 Dean's Research Award to further her research on improving HIV knowledge and training for lactation consultants using telehealth to support people living with HIV in their infant feeding ...
Timed with a summer break or fourth-year research elective Travel to an HIV Vaccine Clinical Research Site in the US or abroad Attend and present at an HVTN Conference Maximum award of $20,000. Project Options. During the application process, scholars will have the opportunity to rank all of the research projects according to their interests.