Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Methodology
- What Is a Cohort Study? | Definition & Examples

What Is a Cohort Study? | Definition & Examples
Published on February 24, 2023 by Tegan George .
A cohort study is a type of observational study that follows a group of participants over a period of time, examining how certain factors (like exposure to a given risk factor) affect their health outcomes. The individuals in the cohort have a characteristic or lived experience in common, such as birth year or geographic area.
While there are several types of cohort study—including open, closed, and dynamic—there are two that are particularly common: prospective cohort studies and retrospective cohort studies .
The initial cohort consisted of about 18,000 newborns. They were enrolled in the study shortly after birth, with regular follow-ups, medical examinations, and cognitive assessments to track their physical, social, and cognitive development.
Cohort studies are particularly useful for identifying risk factors for diseases. They can help researchers identify potential interventions to help prevent or treat the disease, and are often used in fields like medicine or healthcare research.
Table of contents
When to use a cohort study, examples of cohort studies, advantages and disadvantages of cohort studies, frequently asked questions.
Cohort studies are a type of observational study that can be qualitative or quantitative in nature. They can be used to conduct both exploratory research and explanatory research depending on the research topic.
In prospective cohort studies , data is collected over time to compare the occurrence of the outcome of interest in those who were exposed to the risk factor and those who were not. This can help ascertain whether the risk factor could be associated with the outcome.
In retrospective cohort studies , your participants must already possess the disease or health outcome being studied prior to joining. The study is then focused on analyzing the health outcomes of those who share the exposure to the risk factor over a period of time.
A cohort study could be a good fit for your research if:
- You have access to a large pool of research subjects and are comfortable and able to fund research stretching over a longer timeline.
- The relationship between the exposure and health outcome you’re studying is not well understood, and/or its long-term effects have not been thoroughly investigated.
- The exposure you’re studying is rare, or there are possible ethical considerations preventing you from a traditional experimental design .
- Cohort studies in general are more longitudinal in nature. They usually follow the group studied over a long period of time, investigating how certain factors affect their health outcomes.
- Case–control studies rely on primary research , comparing a group of participants already possessing a condition of interest to a control group lacking that condition in real time.
Prevent plagiarism. Run a free check.
Cohort studies are common in fields like medicine, epidemiology, and healthcare.
Cohort studies are a strong research method , particularly in epidemiology, health, and medicine, but they are not without their disadvantages.
Advantages of cohort studies
Advantages of cohort studies include:
- Cohort studies are better able to approach an estimation of causality than other types of observational studies. Due to their ability to establish temporality, multiple outcomes, and disease incidence over time, researchers are able to determine with more certainty that the exposure indeed preceded the outcome. This strengthens a claim for a cause-and-effect relationship between the variables of interest.
- Due to their long nature, cohort studies are a particularly good choice for studying rare exposures , such as exposure to a new drug or an environmental toxin. Other research designs aren’t able to incorporate the breadth and depth of the impact as broadly as cohort studies do.
- Because cohort studies usually rely on large groups of participants, they are better able to control for potentially confounding variables , such as age, gender identity, or socioeconomic status. Relatedly, the ability to use a sampling method that ensures a more representative sample of the population leads to findings that are typically much more generalizable , with higher internal validity and external validity .
Disadvantages of cohort studies
Disadvantages of cohort studies include:
- Cohort studies can be extremely time-consuming and expensive to conduct due to their long and intense nature.
- Cohort studies are at risk for biases inherent to long-term studies like attrition bias and survivorship bias , as participants are likely to drop out over time. Measurement errors like omitted variable bias and information bias can also confound your analysis, leading you to draw conclusions that may not be true.
- Like many other experimental designs , cohort studies can raise questions regarding ethical considerations . This is particularly the case if the exposure of interest is harmful, or if there is no known treatment for it. Prior to beginning your research, it is critical to ensure that participation in your study is fully voluntary, informed, and as safe as it can be for your research subjects.
The easiest way to remember the difference between prospective and retrospective cohort studies is timing.
- A prospective cohort study moves forward in time, following a group of participants to track the development of an outcome of interest.
- A retrospective cohort study moves backward in time, first identifying a group of people who already possess the outcome of interest, and then looking backwards to assess their exposure to a risk factor.
A closed cohort study is a type of cohort study where all participants are selected at the beginning of the study, with no new participants added during any of the follow-up periods.
This approach is useful when the exposure being studied is rare, or when it isn’t practically or financially feasible to recruit new participants.
In a cohort study , the incidence refers to the number of new cases of a disease or health outcome that develop during the study period, while prevalence refers to the proportion of the population who have the disease or health outcome at a given point in time. Cohort studies are particularly useful for measuring incidence rates.
A dynamic cohort study is a type of cohort study where the participants are not fixed at the start of the study. Instead, new participants can be added over time if they become eligible to participate. This approach is useful when the study population is expected to change over time.
Sources in this article
We strongly encourage students to use sources in their work. You can cite our article (APA Style) or take a deep dive into the articles below.
George, T. (2023, February 24). What Is a Cohort Study? | Definition & Examples. Scribbr. Retrieved September 16, 2024, from https://www.scribbr.com/methodology/cohort-study/
Euser, A. M., Zoccali, C., Jager, K. J., & Dekker, F. W. (2009). Cohort Studies: Prospective versus Retrospective. Nephron Clinical Practice , 113 (3), c214–c217. https://doi.org/10.1159/000235241
Is this article helpful?
Tegan George
Other students also liked, what is a prospective cohort study | definition & examples, what is a retrospective cohort study | definition & examples, what is an observational study | guide & examples, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
- En español – ExME
- Em português – EME
Case-control and Cohort studies: A brief overview
Posted on 6th December 2017 by Saul Crandon

Introduction
Case-control and cohort studies are observational studies that lie near the middle of the hierarchy of evidence . These types of studies, along with randomised controlled trials, constitute analytical studies, whereas case reports and case series define descriptive studies (1). Although these studies are not ranked as highly as randomised controlled trials, they can provide strong evidence if designed appropriately.
Case-control studies
Case-control studies are retrospective. They clearly define two groups at the start: one with the outcome/disease and one without the outcome/disease. They look back to assess whether there is a statistically significant difference in the rates of exposure to a defined risk factor between the groups. See Figure 1 for a pictorial representation of a case-control study design. This can suggest associations between the risk factor and development of the disease in question, although no definitive causality can be drawn. The main outcome measure in case-control studies is odds ratio (OR) .
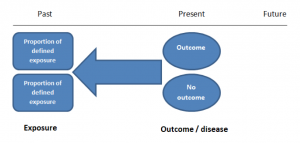
Figure 1. Case-control study design.
Cases should be selected based on objective inclusion and exclusion criteria from a reliable source such as a disease registry. An inherent issue with selecting cases is that a certain proportion of those with the disease would not have a formal diagnosis, may not present for medical care, may be misdiagnosed or may have died before getting a diagnosis. Regardless of how the cases are selected, they should be representative of the broader disease population that you are investigating to ensure generalisability.
Case-control studies should include two groups that are identical EXCEPT for their outcome / disease status.
As such, controls should also be selected carefully. It is possible to match controls to the cases selected on the basis of various factors (e.g. age, sex) to ensure these do not confound the study results. It may even increase statistical power and study precision by choosing up to three or four controls per case (2).
Case-controls can provide fast results and they are cheaper to perform than most other studies. The fact that the analysis is retrospective, allows rare diseases or diseases with long latency periods to be investigated. Furthermore, you can assess multiple exposures to get a better understanding of possible risk factors for the defined outcome / disease.
Nevertheless, as case-controls are retrospective, they are more prone to bias. One of the main examples is recall bias. Often case-control studies require the participants to self-report their exposure to a certain factor. Recall bias is the systematic difference in how the two groups may recall past events e.g. in a study investigating stillbirth, a mother who experienced this may recall the possible contributing factors a lot more vividly than a mother who had a healthy birth.
A summary of the pros and cons of case-control studies are provided in Table 1.
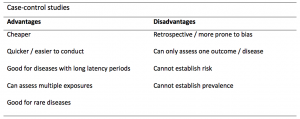
Table 1. Advantages and disadvantages of case-control studies.
Cohort studies
Cohort studies can be retrospective or prospective. Retrospective cohort studies are NOT the same as case-control studies.
In retrospective cohort studies, the exposure and outcomes have already happened. They are usually conducted on data that already exists (from prospective studies) and the exposures are defined before looking at the existing outcome data to see whether exposure to a risk factor is associated with a statistically significant difference in the outcome development rate.
Prospective cohort studies are more common. People are recruited into cohort studies regardless of their exposure or outcome status. This is one of their important strengths. People are often recruited because of their geographical area or occupation, for example, and researchers can then measure and analyse a range of exposures and outcomes.
The study then follows these participants for a defined period to assess the proportion that develop the outcome/disease of interest. See Figure 2 for a pictorial representation of a cohort study design. Therefore, cohort studies are good for assessing prognosis, risk factors and harm. The outcome measure in cohort studies is usually a risk ratio / relative risk (RR).
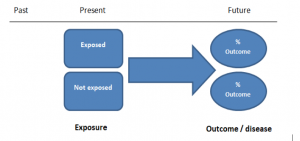
Figure 2. Cohort study design.
Cohort studies should include two groups that are identical EXCEPT for their exposure status.
As a result, both exposed and unexposed groups should be recruited from the same source population. Another important consideration is attrition. If a significant number of participants are not followed up (lost, death, dropped out) then this may impact the validity of the study. Not only does it decrease the study’s power, but there may be attrition bias – a significant difference between the groups of those that did not complete the study.
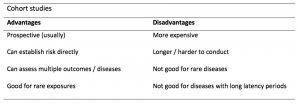
Cohort studies can assess a range of outcomes allowing an exposure to be rigorously assessed for its impact in developing disease. Additionally, they are good for rare exposures, e.g. contact with a chemical radiation blast.
Whilst cohort studies are useful, they can be expensive and time-consuming, especially if a long follow-up period is chosen or the disease itself is rare or has a long latency.
A summary of the pros and cons of cohort studies are provided in Table 2.
The Strengthening of Reporting of Observational Studies in Epidemiology Statement (STROBE)
STROBE provides a checklist of important steps for conducting these types of studies, as well as acting as best-practice reporting guidelines (3). Both case-control and cohort studies are observational, with varying advantages and disadvantages. However, the most important factor to the quality of evidence these studies provide, is their methodological quality.
- Song, J. and Chung, K. Observational Studies: Cohort and Case-Control Studies . Plastic and Reconstructive Surgery.  2010 Dec;126(6):2234-2242.
- Ury HK. Efficiency of case-control studies with multiple controls per case: Continuous or dichotomous data . Biometrics . 1975 Sep;31(3):643–649.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.  Lancet 2007 Oct;370(9596):1453-14577. PMID: 18064739.
Saul Crandon
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on Case-control and Cohort studies: A brief overview
Very well presented, excellent clarifications. Has put me right back into class, literally!
Very clear and informative! Thank you.
very informative article.
Thank you for the easy to understand blog in cohort studies. I want to follow a group of people with and without a disease to see what health outcomes occurs to them in future such as hospitalisations, diagnoses, procedures etc, as I have many health outcomes to consider, my questions is how to make sure these outcomes has not occurred before the “exposure disease”. As, in cohort studies we are looking at incidence (new) cases, so if an outcome have occurred before the exposure, I can leave them out of the analysis. But because I am not looking at a single outcome which can be checked easily and if happened before exposure can be left out. I have EHR data, so all the exposure and outcome have occurred. my aim is to check the rates of different health outcomes between the exposed)dementia) and unexposed(non-dementia) individuals.
Very helpful information
Thanks for making this subject student friendly and easier to understand. A great help.
Thanks a lot. It really helped me to understand the topic. I am taking epidemiology class this winter, and your paper really saved me.
Happy new year.
Wow its amazing n simple way of briefing ,which i was enjoyed to learn this.its very easy n quick to pick ideas .. Thanks n stay connected
Saul you absolute melt! Really good work man
am a student of public health. This information is simple and well presented to the point. Thank you so much.
very helpful information provided here
really thanks for wonderful information because i doing my bachelor degree research by survival model
Quite informative thank you so much for the info please continue posting. An mph student with Africa university Zimbabwe.
Thank you this was so helpful amazing
Apreciated the information provided above.
So clear and perfect. The language is simple and superb.I am recommending this to all budding epidemiology students. Thanks a lot.
Great to hear, thank you AJ!
I have recently completed an investigational study where evidence of phlebitis was determined in a control cohort by data mining from electronic medical records. We then introduced an intervention in an attempt to reduce incidence of phlebitis in a second cohort. Again, results were determined by data mining. This was an expedited study, so there subjects were enrolled in a specific cohort based on date(s) of the drug infused. How do I define this study? Thanks so much.
thanks for the information and knowledge about observational studies. am a masters student in public health/epidemilogy of the faculty of medicines and pharmaceutical sciences , University of Dschang. this information is very explicit and straight to the point
Very much helpful
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

Cluster Randomized Trials: Concepts
This blog summarizes the concepts of cluster randomization, and the logistical and statistical considerations while designing a cluster randomized controlled trial.

Expertise-based Randomized Controlled Trials
This blog summarizes the concepts of Expertise-based randomized controlled trials with a focus on the advantages and challenges associated with this type of study.

An introduction to different types of study design
Conducting successful research requires choosing the appropriate study design. This article describes the most common types of designs conducted by researchers.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Cohort Studies: Design, Analysis, and Reporting
Affiliations.
- 1 Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH. Electronic address: [email protected].
- 2 Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH.
- PMID: 32658655
- DOI: 10.1016/j.chest.2020.03.014
Cohort studies are types of observational studies in which a cohort, or a group of individuals sharing some characteristic, are followed up over time, and outcomes are measured at one or more time points. Cohort studies can be classified as prospective or retrospective studies, and they have several advantages and disadvantages. This article reviews the essential characteristics of cohort studies and includes recommendations on the design, statistical analysis, and reporting of cohort studies in respiratory and critical care medicine. Tools are provided for researchers and reviewers.
Keywords: bias; cohort studies; confounding; prospective; retrospective.
Copyright © 2020 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
PubMed Disclaimer
Similar articles
- A primer on cohort studies in vascular surgery research. Kabeil M, Gillette R, Moore E, Cuff RF, Chuen J, Wohlauer MV. Kabeil M, et al. Semin Vasc Surg. 2022 Dec;35(4):404-412. doi: 10.1053/j.semvascsurg.2022.09.004. Epub 2022 Oct 8. Semin Vasc Surg. 2022. PMID: 36414356 Review.
- [Cohort studies]. Mathis S, Gartlehner G. Mathis S, et al. Wien Med Wochenschr. 2008;158(5-6):174-9. doi: 10.1007/s10354-008-0516-0. Wien Med Wochenschr. 2008. PMID: 18421560 German.
- Ten statistics commandments that almost never should be broken. Knapp TR, Brown JK. Knapp TR, et al. Res Nurs Health. 2014 Aug;37(4):347-51. doi: 10.1002/nur.21605. Epub 2014 Jun 29. Res Nurs Health. 2014. PMID: 24976481
- A Practical Overview of Case-Control Studies in Clinical Practice. Dey T, Mukherjee A, Chakraborty S. Dey T, et al. Chest. 2020 Jul;158(1S):S57-S64. doi: 10.1016/j.chest.2020.03.009. Chest. 2020. PMID: 32658653 Review.
- Cross-Sectional Studies: Strengths, Weaknesses, and Recommendations. Wang X, Cheng Z. Wang X, et al. Chest. 2020 Jul;158(1S):S65-S71. doi: 10.1016/j.chest.2020.03.012. Chest. 2020. PMID: 32658654 Review.
- Elucidating the dynamics and impact of the gut microbiome on maternal nutritional status during pregnancy, effect on pregnancy outcomes and infant health in rural Pakistan: study protocol for a prospective, longitudinal observational study. Wasan Y, Baxter JB, Spiegel-Feld C, Begum K, Rizvi A, Iqbal J, Hulst J, Bandsma R, Suleman S, Soofi S, Parkinson J, Bhutta ZA. Wasan Y, et al. BMJ Open. 2024 Aug 12;14(8):e081629. doi: 10.1136/bmjopen-2023-081629. BMJ Open. 2024. PMID: 39134435 Free PMC article.
- Patient Characteristics and Outcomes of Hospitalized Chronic Kidney Disease Patients with and without Type 2 Diabetes Mellitus: Observations from the German Claims Data-Based Cohort of the CaReMe-CKD Multinational Study. Leiner J, Pellissier V, König S, Stellmacher L, Hohenstein S, Schanner C, Kwast S, Kuhlen R, Bollmann A. Leiner J, et al. Clin Epidemiol. 2024 Jul 22;16:487-500. doi: 10.2147/CLEP.S459767. eCollection 2024. Clin Epidemiol. 2024. PMID: 39070102 Free PMC article.
- Methodological and Statistical Considerations for Cross-Sectional, Case-Control, and Cohort Studies. Pérez-Guerrero EE, Guillén-Medina MR, Márquez-Sandoval F, Vera-Cruz JM, Gallegos-Arreola MP, Rico-Méndez MA, Aguilar-Velázquez JA, Gutiérrez-Hurtado IA. Pérez-Guerrero EE, et al. J Clin Med. 2024 Jul 9;13(14):4005. doi: 10.3390/jcm13144005. J Clin Med. 2024. PMID: 39064045 Free PMC article. Review.
- Role of C1q/TNF-Related Protein 6 for the Evaluation of Coronary Heart Disease Associated with Type 2 Diabetes [Letter]. Edi IS, Luthfiyah S, Triwiyanto T, Utomo B. Edi IS, et al. Ther Clin Risk Manag. 2024 Jul 17;20:449-450. doi: 10.2147/TCRM.S481485. eCollection 2024. Ther Clin Risk Manag. 2024. PMID: 39050409 Free PMC article. No abstract available.
- Association between p16/Ki-67 dual stain cytology results prior to and 6 months after LLETZ treatment for CIN and the follow-up regimen three years after treatment: a retrospective cohort study. Packet B, Goyens J, Weynand B, Poppe W, Dewilde K. Packet B, et al. Arch Gynecol Obstet. 2024 Jul;310(1):493-499. doi: 10.1007/s00404-024-07553-8. Epub 2024 May 28. Arch Gynecol Obstet. 2024. PMID: 38806944
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- ClinicalKey
- Elsevier Science
- Ovid Technologies, Inc.
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Dermatol
- v.61(1); Jan-Feb 2016
Methodology Series Module 1: Cohort Studies
Maninder singh setia.
From the Department of Epidemiology, MGM Institute of Health Sciences, Navi Mumbai, Maharashtra, India
Cohort design is a type of nonexperimental or observational study design. In a cohort study, the participants do not have the outcome of interest to begin with. They are selected based on the exposure status of the individual. They are then followed over time to evaluate for the occurrence of the outcome of interest. Some examples of cohort studies are (1) Framingham Cohort study, (2) Swiss HIV Cohort study, and (3) The Danish Cohort study of psoriasis and depression. These studies may be prospective, retrospective, or a combination of both of these types. Since at the time of entry into the cohort study, the individuals do not have outcome, the temporality between exposure and outcome is well defined in a cohort design. If the exposure is rare, then a cohort design is an efficient method to study the relation between exposure and outcomes. A retrospective cohort study can be completed fast and is relatively inexpensive compared with a prospective cohort study. Follow-up of the study participants is very important in a cohort study, and losses are an important source of bias in these types of studies. These studies are used to estimate the cumulative incidence and incidence rate. One of the main strengths of a cohort study is the longitudinal nature of the data. Some of the variables in the data will be time-varying and some may be time independent. Thus, advanced modeling techniques (such as fixed and random effects models) are useful in analysis of these studies.
Introduction
Cohort studies are important in research design. The term “cohort” is derived from the Latin word “ Cohors ” – “a group of soldiers.” It is a type of nonexperimental or observational study design. The term “cohort” refers to a group of people who have been included in a study by an event that is based on the definition decided by the researcher. For example, a cohort of people born in Mumbai in the year 1980. This will be called a “birth cohort.” Another example of the cohort will be people who smoke. Some other terms which may be used for these studies are “prospective studies” or “longitudinal studies.”
In a cohort study, the participants do not have the outcome of interest to begin with. They are selected based on the exposure status of the individual. Thus, some of the participants may have the exposure and others do not have the exposure at the time of initiation of the study. They are then followed over time to evaluate for the occurrence of the outcome of interest.
As seen in Figure 1 , at baseline, some of the study participants have exposure (defined as exposed) and others do not have the exposure (defined as unexposed). Over the period of follow-up, some of the exposed individuals will develop the outcome and some unexposed individuals will develop the outcome of interest. We will compare the outcomes in these two groups.

Example of a cohort study
Examples of Cohort Studies
Framingham cohort study ( https://www.framinghamheartstudy.org/index.php ).
This cohort study was initiated in 1948 in Framingham. Framingham, at the time of initiation of the cohort, was an industrial town 21 miles west of Boston with a population of 28,000. This Framingham Heart Study recruited 5209 men and women (30–62-year-old) in the study to assess the factors associated with cardiovascular disease (CVD). The researchers also recruited second generation participants (children of original participants) in 1971 and the third general participants in 2002. This has been one of the landmark cohort studies and has contributed immensely to our knowledge of some of the important risk factors for CVD. The investigators have published 3064 publications using the Framingham Heart Study data.
Swiss HIV cohort study ( http://www.shcs.ch/ )
This cohort study was initiated in 1988. It was a longitudinal study of HIV-infected individuals to conduct research on HIV pathogenesis, treatment, immunology, and coinfections. They also work on the social aspects of the disease and management of HIV-infected pregnant women. The study started with a recruitment of individuals ≥16 years. The cohort was gradually expanded to include the Swiss Mother and Child HIV Cohort Study. The cohort has provided useful information on various aspects of HIV and published 542 manuscripts on these aspects.
The Danish cohort study of psoriasis and depression (Jensen, 2015)
This is another large cohort study that evaluated the association between psoriasis and onset of depression. The participants in the cohort were enrolled from national registries in Denmark. None of the included participants had psoriasis or depression at baseline. The outcome of interest was the initiation of antidepressants or hospitalization for depression. The authors compared the incidence rates of hospitalization for depression in psoriasis and reference population. The psoriasis group was further classified as mild and moderate psoriasis. The authors found that psoriasis was an independent risk factor for new-onset depression in young people. However, in the elderly, it was mediated through comorbid conditions.
We have presented examples of some large cohort studies. It will be worthwhile to read the design and conduct of these studies, and it will help the readers understand the practical aspects of conducting and analyzing cohort studies.
Types of Cohort Studies
Prospective cohort study.
In this type of cohort study, all the data are collected prospectively. The investigator defines the population that will be included in the cohort. They then measure the potential exposure of interest. The participants are then classified as exposed or unexposed by the investigator. The investigator then follows these participants. At baseline and during follow-up, the investigator also collects information on other variables that are important for the study (such as confounding variables). The investigator then assesses the outcome of interest in these individuals. Some of these outcomes may only occur once (for example, death), and some may occur multiple times (for example, conditions which may recur in the same individual – diarrhea, wheezing episodes, etc.).
Retrospective cohort study
In this type of cohort study, the data are collected from records. Thus, the outcomes have occurred in the past. Even though the outcomes have occurred in the past, the basic study design is essentially the same. Thus, the investigator starts with the exposure and other variables at baseline and at follow-up and then measures the outcome during the follow-up period.
Sometimes, the direction may not be as well defined as prospective and retrospective. One may analyze retrospective data on a group of people well as collect prospective data from the same individuals.
Examples of prospective and retrospective cohort studies
Our objective is to estimate the incidence of cardiovascular events in patients with psoriasis. We have decided to conduct a 10-year study. All the individuals who are diagnosed with psoriasis are eligible for being included in this cohort study. However, one has to ensure that none of them have cardiovascular events at baseline. Thus, they should be thoroughly investigated for the presence of these events at baseline before including them in the study. For this, we have to define all the events we are interested in the study (such as angina or myocardial infarction). The criteria for identifying psoriasis and cardiovascular outcomes should be decided before initiating the study. All those who do not have cardiovascular outcomes should be followed at regular intervals (predecided by the researcher and as required for clinical management). This will be a prospective cohort study.
Our objective is to assess the survival in HIV-infected individuals and the factors associated with survival. We have clinical data from about 430 HIV-infected individuals in the center. The follow-up period ranges from 3 months to 4 years, and we know that 33 individuals have died in this group. We decide to perform the survival analysis in this group of individuals. We prepare a clinical record form and abstract data from these clinical forms. This design will be a retrospective cohort study.
Outcomes in a Cohort Study
A cohort study may have different types of outcomes. Some of the outcomes may occur only once. In the above mentioned retrospective study, if we assess the mortality in these individuals, then the outcome will occur only once. Other outcomes in the cohort study may be measured more than once. For instance, if we assess CD4 counts in the same retrospective study, then the values of CD4 counts may change at every visit. Thus, the outcome will be measured at every visit.
Strengths of a Cohort Study
- Temporality: Since at the time of entry into the cohort study, the individuals do not have outcome, the temporality between exposure and outcome is well defined
- A cohort study helps us to study multiple outcomes in the same exposure. For example, if we follow patients of hypercholesterolemia, we can study the incidence of melasma or psoriasis in them. Thus, there is one exposure (hypercholesterolemia) and multiple outcomes (melasma and psoriasis). However, we have to ensure that none of the individuals have any of the outcomes at the baseline
- If the exposure is rare, then a cohort design is an efficient method to study the relation between exposure and outcomes
- It is generally said that a cohort design may not be efficient for rare outcomes (a case-control design is preferred). However, if the rare outcome is common in some exposures, then it may be useful to follow a cohort design. For example, melanoma is not a common condition in India. Hence, if we follow individuals to study the incidence of melanoma, then it may not be efficient. However, if we know that, theoretically, a particular chemical may be associated with melanoma, then we should follow a cohort of individuals exposed to this chemical (in occupational settings or otherwise) and study the incidence of melanoma in this group
- In a prospective cohort study, the exposure variable, other variables, and outcomes may be measured more accurately. This is important to maintain uniformity in the measurement of exposures and outcomes. This is also useful for exposures that may require subjective assessment or recall by the patient. For example, dietary history, smoking history, or alcoholic history, etc. This may help in reducing the bias in measurement of exposure
- A retrospective cohort study can be completed fast and is relatively inexpensive compared with a prospective cohort study. However, it also has other strengths of the prospective cohort study.
Limitations of a Cohort Study
- One major limitation of a prospective cohort design is that is time consuming and costly. For example, if we have to study the incidence of cardiovascular patients in patients of psoriasis, we may have to follow them up for many years before the outcome occurs
- In a retrospective cohort study, the exposure and the outcome variables are collected before the study has been initiated. Thus, the measurements may not be very accurate or according to our requirements. In addition, the some of the exposures may have been assessed differently for various members of the cohort
- As discussed earlier, cohort studies may not be very efficient for rare outcomes except in some conditions.

Additional Points in Cohort Studies
Multiple cohort study.
Sometimes, we may be interested to compare the outcomes in two or more groups of individuals. Thus, we may have a multiple cohort study. It is important the exposure, outcome, and other variables should be measured similarly in both the study and the comparison group.
Measurement of exposure and outcome
Since the individuals are included in the study based on the exposure status, this has to be well defined and accurate. The outcomes also have to be well defined and measured similarly in all the participants. If you have more than one group in the cohort (as in multiple cohorts or reference population), you should ensure that the follow-up protocols are similar in all the groups.
Question: What if there is an error in measuring the exposure or the outcome?
It is quite possible that individuals participating in a cohort study may not be correctly classified – some exposed individuals may be classified as unexposed and the other way round. If the misclassification of the exposure or the outcome is random or nondifferential, then the two groups will be similar and the estimates from the study will be biased towards the null. Thus, we will underestimate the association between the exposure and the outcome. If, however, the misclassification is differential or nonrandom, then the estimates may be biased toward the null, away from the null, or may be an appropriate estimate.
Follow-up of the study participants is very important in a cohort study and losses are an important source of bias in these types of studies. Some patients are lost to follow-up in large cohorts; however, if the proportion is very high (>30%), then the validity of the results from this study are doubtful. This loss to follow-up becomes all the more important if it is related to the exposure or outcome of interest. For example, in our prospective study, majority of the patients who were lost to follow-up had severe psoriasis at the baseline, then we will get biased estimates from the study. Thus, managing follow-ups and minimizing losses are an important component of the design of a cohort study.
Nested case-control study
This is a specific type of study design nested within a cohort study. In this, the investigator will match the controls to the cases within a specific cohort. The exposure of interest will be assessed in these selected cases and controls. For example, our hypothesis is that there is a biological marker that in present/elevated (to begin with) in individuals who develop cardiovascular events in psoriatic patients. It is expensive to assess this marker in all patients. Thus, we select all those who develop the outcomes (cases) in our cohort and a sample of individuals who do not develop the outcomes (controls). An important aspect, however, is that we should have stored the biological material that we have collected at baseline, and the biological marker should be assessed in this sample. This procedure maintains the temporal strength of the cohort study.
Cohort studies will help us to estimate the cumulative incidence and incidence rate.
Cumulative incidence
We follow 10,000 psoriatic patients for 10 years. Of these, 50 have a cardiovascular event. Thus, the cumulative incidence will be 50/10,000 or 0.005. This measure is a proportion. Thus, the cumulative incidence will be 0.5% or 5/1000.
Incidence rate
We follow-up 10,000 psoriatic patients for 10 years. Of these, 50 have a cardiovascular event.
How do we calculate the incidence rate?
Let us assume that all the cardiovascular events occurred at the end of the 2 nd year. Our outcome of interest was the first cardiovascular event. Thus, at the end of the 2 nd year, 50 individuals have the outcome.
The total time contributed by these 50 individuals is 50 × 2 years = 100 person years (PY) - (A).
The total time contributed by the rest of the cohort is (10,000 − 50) × 10 = 99,500 PY - (B).
Thus, the total person time is A + B = 99,600.
The incidence rate is 50/99,600 or 0.000502. As it is obvious from the term, this measure is a rate (compared with cumulative incidence which was a proportion). Thus, the incidence rate of first cardiovascular event in psoriatic patients is 0.502/1000 PY or 5.02/10,000 PY.
Other analysis
Other methods such as logistic regression, Kalpan–Meier curves, cox-regression, Poisson regression, lognormal regression may be useful in cohort studies. These are relatively advanced analyses and should be discussed with a statistician.
Fixed and random effects models
One of the main strengths of a cohort study is the longitudinal nature of the data. Some of the variables are time varying (such as blood pressure), and some may be time independent (such as sex). The fixed and random effects models are useful to handle longitudinal data. The random effects model provides both between- and within-individual variance and is useful for time-dependent and time-independent variables. These models are used in linear outcomes (such as body mass index) or categorical outcomes (such as presence/absence of psoriasis). These are advanced modeling techniques and should be discussed with a statistician.
Some Practical Points
Project management.
The investigator should remember that conducting a large-scale prospective cohort study requires proper project management.
Follow-up of participants
The investigator should devise strategies to ensure proper follow-up of individuals at the designated time intervals. A computer program should be put in place at the start of the prospective study. The program should indicate the number of participants due for a visit every day. If the individual does not visit for the next week, a reminder should be sent to the individual. This can be performed through texting or a phone call to the individual. Some investigators hire field workers or outreach workers to ensure follow-up of study participants.
It is important that we include only patients with permanent addresses in the area for long-term cohort studies. Details about the stay (permanent address, temporary address, and duration of residence in the current address) should be a part of the inclusion criteria.
Data management
The investigator should prioritize data management in these studies. The data entry program should be installed at the start of the project. In addition, data entry and cleaning should be done as soon as data are collected. This will help us to identify the lacunae in the existing data, loss of follow-ups, and missing data points.
Missing data
It is very important to address missing data in cohort studies. There are statistical methods to handle missing data in studies – such as complete case analysis, available case analysis, single imputation, or multiple imputations. The investigator should work with a statistician to address missing data in the dataset. These methods should also be described in the statistical analysis section of the manuscript.
In a cohort study, participants who do not have the outcome at baseline are followed over time to estimate the incidence of the outcome. In this type of design, the temporality between the exposure and outcome is well defined. The studies may be prospective, retrospective, or a mixture of both. Prospective cohort studies may be time consuming and expensive. Losses during follow-up are an important source of bias in cohort studies; thus, measures to ensure follow-up of participants should be included in the design of a prospective cohort study. Advanced modeling techniques are useful to analyze longitudinal data and are preferred in cohort studies.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
Bibliography
Cohort Study: Definition, Designs & Examples
Julia Simkus
Editor at Simply Psychology
BA (Hons) Psychology, Princeton University
Julia Simkus is a graduate of Princeton University with a Bachelor of Arts in Psychology. She is currently studying for a Master's Degree in Counseling for Mental Health and Wellness in September 2023. Julia's research has been published in peer reviewed journals.
Learn about our Editorial Process
Saul McLeod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul McLeod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
A cohort study is a type of longitudinal study where a group of individuals (cohort), often sharing a common characteristic or experience, is followed over an extended period of time to study and track outcomes, typically related to specific exposures or interventions.
In cohort studies, the participants must share a common factor or characteristic such as age, demographic, or occupation. A “cohort” is a group of subjects who share a defining characteristic.
Cohort studies are observational, so researchers will follow the subjects without manipulating any variables or interfering with their environment.
This type of study is beneficial for medical researchers, specifically in epidemiology, as scientists can use data from cohort studies to understand potential risk factors or causes of a disease.
Before any appearance of the disease is investigated, medical professionals will identify a cohort, observe the target participants over time, and collect data at regular intervals.
Weeks, months, or years later, depending on the duration of the study design, the researchers will examine any factors that differed between the individuals who developed the condition and those who did not.
They can then determine if an association exists between an exposure and an outcome and even identify disease progression and relative risk.
Retrospective
- A retrospective cohort study is a type of observational research that uses existing past data to identify two groups of individuals—those with the risk factor or exposure (cohort) and without—and follows their outcomes backward in time to determine the relationship.
- In a retrospective study , the subjects have already experienced the outcome of interest or developed the disease before starting the study.
- The researchers then look back in time to identify a cohort of subjects before developing the disease and use existing data, such as medical records, to discover any patterns.
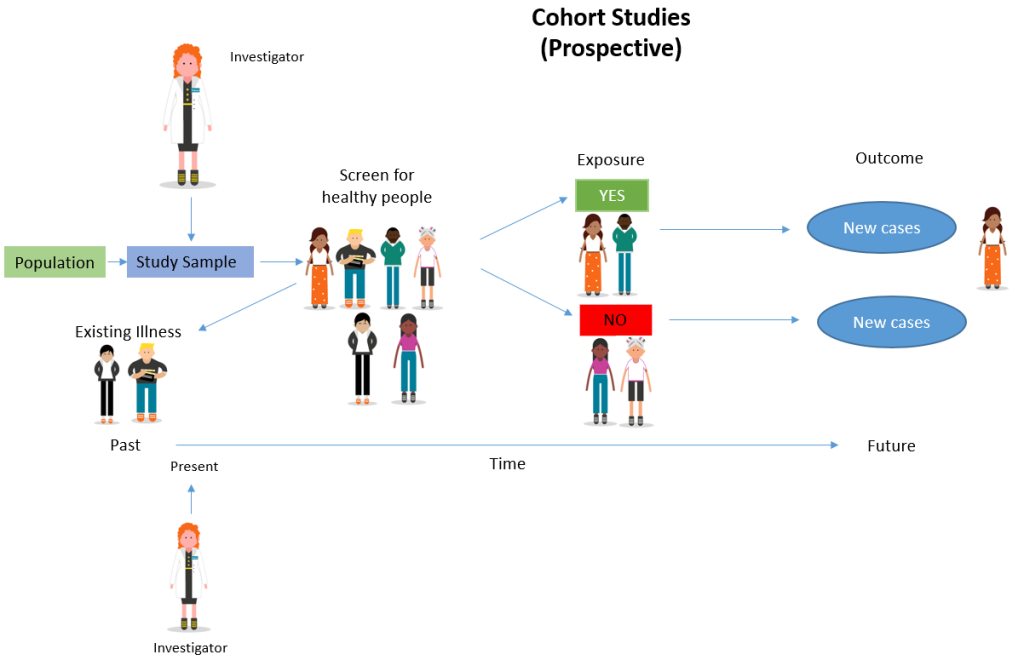
Prospective
A prospective cohort study is a type of longitudinal research where a group of individuals sharing a common characteristic (cohort) is followed over time to observe and measure outcomes, often to investigate the effect of suspected risk factors.
In a prospective study , the investigators will design the study, recruit subjects, and collect baseline data on all subjects before they have developed the outcomes of interest.
- The subjects are followed and observed over a period of time to gather information and record the development of outcomes.
Determine cause-and-effect relationships
Because researchers study groups of people before they develop an illness, they can discover potential cause-and-effect relationships between certain behaviors and the development of a disease.
Provide extensive data
Cohort studies enable researchers to study the causes of disease and identify multiple risk factors associated with a single exposure. These studies can also reveal links between diseases and risk factors.
Enable studies of rare exposures
Cohort studies can be very useful for evaluating the effects and risks of rare diseases or unusual exposures, such as toxic chemicals or adverse effects of drugs.
Can measure a continuously changing relationship between exposure and outcome
Because cohort studies are longitudinal, researchers can study changes in levels of exposure over time and any changes in outcome, providing a deeper understanding of the dynamic relationship between exposure and outcome.
Limitations
Time consuming and expensive.
Cohort studies usually require multiple months or years before researchers are able to identify the causes of a disease or discover significant results. Because of this, they are often more expensive than other types of studies. Retrospective studies, though, tend to be cheaper and quicker than prospective studies as the data already exists.
Require large sample sizes
Cohort studies require large sample sizes in order for any relationships or patterns to be meaningful. Researchers are unable to generate results if there is not enough data.
Prone to bias
Because of the longitudinal nature of these studies, it is common for participants to drop out and not complete the study. The loss of follow-up in cohort studies means researchers are more likely to estimate the effects of an exposure on an outcome incorrectly.
Unable to discover why or how a certain factor is associated with a disease
Cohort studies are used to study cause-and-effect relationships between a disease and an outcome. However, they do not explain why the factors that affect these relationships exist. Experimental studies are required to determine why a certain factor is associated with a particular outcome.
The Framingham Heart Study
Studied the effects of diet, exercise, and medications on the development of hypertensive or arteriosclerotic cardiovascular disease, in a longitudinal population-based cohort.
The Whitehall Study
The initial prospective cohort study examined the association between employment grades and mortality rates of 17139 male civil servants over a period of ten years, beginning in 1967. When the Whitehall Study was conducted, there was no requirement to obtain ethical approval for scientific studies of this kind.
The Nurses’ Health Study
Researched long-term effects of nurses” nutrition, hormones, environment, and work-life on health and disease development.
The British Doctors Study
This was a prospective cohort study that ran from 1951 to 2001, investigating the association between smoking and the incidence of lung cancer.
The Black Women’s Health Study
Gathered information about the causes of health problems that affect Black women.
Millennium Cohort Study
Found evidence to show how various circumstances in the first stages of life can influence later health and development. The study began with an original sample of 18,818 cohort members.
The Danish Cohort Study of Psoriasis and Depression
Studied the association between psoriasis and the onset of depression.
The 1970 British Cohort Study
Followed the lives of around 17,000 people born in England, Scotland, and Wales in a single week of 1970.
Frequently Asked Questions
1. are case-control studies and cohort studies the same.
While both studies are commonly used among medical professionals to study disease, they differ.
Case-control studies are performed on individuals who already have a disease (cases) and compare them with individuals who share similar characteristics but do not have the disease (controls).
In cohort studies, on the other hand, researchers identify a group before any of the subjects have developed the disease. Then after an extended period, they examine any factors that differed between the individuals who developed the condition and those who did not.
2. What is the difference between a cross-sectional study and a cohort study?
Like case-control and cohort studies, cross-sectional studies are also used in epidemiology to identify exposures and outcomes and compare the rates of diseases and symptoms of an exposed group with an unexposed group.
However, cross-sectional studies analyze information about a population at a specific point in time, while cohort studies are carried out over longer periods.
3. What is the difference between cohort and longitudinal studies?
A cohort study is a specific type of longitudinal study. Another type of longitudinal study is called a panel study which involves sampling a cross-section of individuals at specific intervals for an extended period.
Panel studies are a type of prospective study, while cohort studies can be either prospective or retrospective.
Barrett D, Noble H. What are cohort studies? Evidence-Based Nursing 2019; 22:95-96.
Kandola, A.A., Osborn, D.P.J., Stubbs, B. et al. Individual and combined associations between cardiorespiratory fitness and grip strength with common mental disorders: a prospective cohort study in the UK Biobank. BMC Med 18, 303 (2020). https://doi.org/10.1186/s12916-020-01782-9
Marmot, M. G., Rose, G., Shipley, M., & Hamilton, P. J. (1978). Employment grade and coronary heart disease in British civil servants. Journal of Epidemiology & Community Health, 32(4), 244-249.
Rosenberg, L., Adams-Campbell, L., & Palmer, J. R. (1995). The Black Women’s Health Study: a follow-up study for causes and preventions of illness. Journal of the American Medical Women’s Association (1972), 50(2), 56-58.
Samer Hammoudeh, Wessam Gadelhaq and Ibrahim Janahi (November 5th 2018). Prospective Cohort Studies in Medical Research, Cohort Studies in Health Sciences, R. Mauricio Barría, IntechOpen, DOI: 10.5772/intechopen.76514. Available from: https://www.intechopen.com/chapters/60939
Setia M. S. (2016). Methodology Series Module 1: Cohort Studies. Indian journal of dermatology, 61(1), 21–25. https://doi.org/10.4103/0019-5154.174011
Zabor, E. C., Kaizer, A. M., & Hobbs, B. P. (2020). Randomized Controlled Trials. Chest, 158(1). https://doi.org/10.1016/j.chest.2020.03.013
Further Information
- Cohort Effect? Definition and Examples
- Barrett, D., & Noble, H. (2019). What are cohort studies?. Evidence-based nursing, 22(4), 95-96.
- The Whitehall Studies
- Euser, A. M., Zoccali, C., Jager, K. J., & Dekker, F. W. (2009). Cohort studies: prospective versus retrospective. Nephron Clinical Practice, 113(3), c214-c217.

IMAGES
VIDEO
COMMENTS
Cohort studies are a type of observational study that can be qualitative or quantitative in nature. They can be used to conduct both exploratory research and explanatory research depending on the research topic.
Since cohort studies are observational, study participants are monitored, and study interventions are not provided. This paper describes the prospective and retrospective cohort designs, examines the strengths and weaknesses, and discusses methods to report the results.
Cohort studies are, therefore, empirical, longitudinal studies based on data obtained from a sample; they are also observational and (usually) naturalistic. Analyses can be conducted for the cohort as a whole or for subgroups amongst which comparisons can be drawn.
Cohort studies are a type of research design. They are also called longitudinal studies because they follow groups of people over time. Results from cohort studies can help people...
Case-control and cohort studies are observational studies that lie near the middle of the hierarchy of evidence. These types of studies, along with randomised controlled trials, constitute analytical studies, whereas case reports and case series define descriptive studies (1).
Cohort studies are types of observational studies in which a cohort, or a group of individuals sharing some characteristic, are followed up over time, and outcomes are measured at one or more time points. Cohort studies can be classified as prospective or retrospective studies, and they have several advantages and disadvantages.
Cohort studies are the design of choice for determining the incidence and natural history of a condition. Due to their longitudinal design feature, one can look at disease progression and natural history. 3 Cohort studies allow us to calculate the incidence rate, cumulative incidence, relative risk, and hazard ratio.
In a cohort study, participants who do not have the outcome at baseline are followed over time to estimate the incidence of the outcome. In this type of design, the temporality between the exposure and outcome is well defined. The studies may be prospective, retrospective, or a mixture of both.
Cohort studies enable researchers to study the causes of disease and identify multiple risk factors associated with a single exposure. These studies can also reveal links between diseases and risk factors. Enable studies of rare exposures.
Cohort studies are types of observational studies in which a cohort, or a group of individuals sharing some characteristic, are followed up over time, and outcomes are measured at one or more time points. Cohort studies can be classified as prospective or retrospective studies, and they have several advantages and disadvantages.